Volume 27, Issue 4 (Autumn 2021)
Intern Med Today 2021, 27(4): 486-501 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Asgharzadeh F, Hosseini M, Beheshti F, Rakhshandeh H, Mansouri S, Anaeigoudari A. Improving Effect of Ethyl Acetate Fraction of Tanacetum Parthenium Against Brain Oxidative Damage in Pentylenetetrazole-Induced Seizure Model in Mice. Intern Med Today 2021; 27 (4) :486-501
URL: http://imtj.gmu.ac.ir/article-1-3603-en.html
URL: http://imtj.gmu.ac.ir/article-1-3603-en.html
Fereshteh Asgharzadeh1 

 , Mahmoud Hosseini2
, Mahmoud Hosseini2 

 , Farimah Beheshti3
, Farimah Beheshti3 

 , Hassan Rakhshandeh1
, Hassan Rakhshandeh1 

 , Somaieh Mansouri4
, Somaieh Mansouri4 

 , Akbar Anaeigoudari *
, Akbar Anaeigoudari * 

 5
5


 , Mahmoud Hosseini2
, Mahmoud Hosseini2 

 , Farimah Beheshti3
, Farimah Beheshti3 

 , Hassan Rakhshandeh1
, Hassan Rakhshandeh1 

 , Somaieh Mansouri4
, Somaieh Mansouri4 

 , Akbar Anaeigoudari *
, Akbar Anaeigoudari * 

 5
5
1- Medicinal Plants Pharmacology Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
2- Psychiatric and Behavioral Sciences Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Applied Biomedical Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
4- Department of Anatomy, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
5- Department of Physiology, School of Medicine, Jiroft University of Medical Sciences, Jiroft, Iran. , anaeiga317@gmail.com
2- Psychiatric and Behavioral Sciences Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Applied Biomedical Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
4- Department of Anatomy, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
5- Department of Physiology, School of Medicine, Jiroft University of Medical Sciences, Jiroft, Iran. , anaeiga317@gmail.com
Full-Text [PDF 4951 kb]
(611 Downloads)
| Abstract (HTML) (1454 Views)
Full-Text: (887 Views)
1. Introduction
Epilepsy is one of the most common neurological disorders that affect many people worldwide [1]. Epilepsy is characterized by reversible spontaneous seizures caused by an imbalance between irritability and inhibition of the brain with a tendency toward uncontrolled irritability [2]. Epilepsy can be divided into two main types: 1) epilepsy of the middle part of the temporal lobe, which affects the hippocampus, parahippocampus, and amygdala, 2) lateral lobe epilepsy, which affects the neocortex [3]. Experimental models of epilepsy induction were used to study the pathophysiology of epileptic seizures and play an essential role in our better understanding of the molecular mechanisms associated with seizure progression [4]. One of the animal models for studying epileptic seizures is the model in which Pentylenetetrazole (PTZ) is used [5]. Increased production of free radicals plays an important role in the pathogenesis of a wide range of neurological disorders, including epilepsy [6]. The hypersensitivity of the brain to oxidative damage highlights the importance of knowing the role of oxidative stress in the pathogenesis of seizures and epilepsy [7]. Scientific evidence also suggests an association between long-term seizures and oxidative damage to lipids, DNA, and proteins [8]. In addition, neurotoxicity induced by some neurotransmitters, including glutamate, is one of the main mechanisms of neurological seizure disorder [9]. For example, researchers have reported that neurotoxicity due to glutamate overactivity leads to overproduction of nitric oxide by the enzyme nitric oxide synthase, resulting in neuronal damage induced by oxidative stress [10]. Scientific evidence also shows that oxidative damage to biomolecules plays an essential role in neuronal hyperstimulation, neuronal damage, and induction of seizures [11]. The results of recent studies confirm that dysfunction of mitochondria as organs affecting oxidative phosphorylation and acute oxidative stress can predispose the brain to epileptic seizures [12]. Scientific reports also indicate the role of oxidative stress in neuronal damage in a model of epilepsy induced by PTZ [13].
Previous experimental studies show that natural compounds in plant extracts are good sources for relieving seizures and reducing their effects in epileptic patients [14]. Tanacetum parthenium belongs to the Cassian family and is widely grown worldwide [15]. Parthenolide is one of the most important compounds in the extract of this plant with anti-migraine properties [16]. In addition, the extract of this plant is used in traditional medicine to reduce fever, relieve asthma, and reduce inflammation [17]. Scientific results show that the polyphenolic compounds and flavonoids in the extract of Tanacetum parthenium have anti-oxidant properties [18]. The researchers reported that the alcoholic extract of this plant has anti-oxidant activity against diphenyl-2-picrylhydrazyl by neutralizing free radicals [19]. The protective effects of this plant extract against kidney damage caused by carbon tetrachloride by improving the state of oxidative stress have also been documented [15]. Also, the anti-oxidant effects of this plant extract against liver damage caused by excessive production of free radicals along with lowering cholesterol and triglycerides have been shown [20]. The results of our previous study showed that the moderate dose (100 mg/kg) of hydroalcoholic extract of Tanacetum parthenium had the best effect against PTZ-induced seizures in mice by decreasing malondialdehyde levels and increasing levels of tom thiols and the anti-oxidant enzymes superoxide dismutase and catalase. In contrast, the n-butanol and aqueous fractions could not improve the oxidative brain damage caused by PTZ-induced seizures [21]. Therefore, compounds other than soluble compounds in n-butanol and aqueous solvents of this plant may have a role in reducing oxidative brain damage caused by pentylenetetrazole-induced seizures. Therefore, to clarify the issue in this study, the effects of ethyl acetate fraction of Tanacetum parthenium plant against oxidative brain damage in a model of PTZ-induced seizures in rats were investigated.
2. Materials and Methods
Preparation of Ethyl Acetate Fraction
To prepare the ethyl acetate fraction, 50 g of plant powder was dissolved in 300 mL of ethanol and extracted using an extractor. Next, 10 g of dried ethanolic extract was dissolved in 50 mL of distilled water and poured into a decanter. Then 50 mL of n-butanol solvent was added to it. In this stage, two phases are formed; the upper phase contains soluble compounds in butanol. After separating the two phases from each other, to prepare the ethyl acetate fraction, the lower phase of the previous step was poured into the decanter, and 50 mL of ethyl acetate solvent was added to it. Two phases are formed again, the upper phase containing ethyl acetate-soluble compounds. After separation, the resulting solution was dried by an incubator and stored for use [21].
Animals and Groups
This study is an experimental study in which 60 male mice weighing 30±5 g were randomly divided into 6 groups of 10 as follows: 1) control group, 2) pentylenetetrazole (PTZ) group, 3) PTZ + fraction with a dose of 25 mg/kg (Fraction25 + PTZ), 4) PTZ + fraction with a dose of 50 mg/kg (Fraction50 + PTZ), 5) PTZ + fraction with a dose of 100 mg/kg (Fraction100 + PTZ), and 6) PTZ + fraction at a dose of 200 mg/kg (Fraction200 + PTZ). The ethyl acetate fraction was injected intraperitoneally for three weeks 30 minutes before pentylenetetrazole. Animals in the control group also received saline. The animals were kept under standard conditions (temperature 22°C-24°C, light period 12 hours of light and 12 hours of darkness, humidity 50%, and adequate access to water and food). The dose of drugs in this study was selected based on previous studies [21]. Before the beginning of the experiments, the proposal of this plan was presented in the Ethics Committee of Mashhad University of Medical Sciences. The ethical code (IR.MUMS.fm.REC.1397.134) belongs to the Ethics Committee of Mashhad University of Medical Sciences.
Induction of seizures by pentylenetetrazole
To induce seizures in mice, pentylenetetrazole (100 mg/kg body weight) was injected intraperitoneally [22, 23]. The animals were then placed in a Plexiglas box, and their convulsive behaviors were assessed for 60 min based on the Becker 5-point method: stage 0, no response; stage 1, face and ear muscle impulses; stage 2, myoclonic impulses without standing on two legs; stage 3, myoclonic impulses with standing on two legs; stage 4, falling to one side and tonic-clonic seizures; and stage 5, falling on the back and generalized tonic-clonic seizures. If stages 4 and 5 were observed, the animal was considered convulsive [24]. Acceleration of the onset of clonic seizures (minimal clonic seizure [MCS]) and generalized tonic-clonic seizures (GTCS) were considered the criteria of seizure severity. After behavioral experiments, the animals were killed, their brains were removed, and the hippocampal and cortical tissues were isolated. Brain tissues were homogenized in 1 mL of phosphate buffer. The homogenized tissue was then centrifuged at 3000 rpm for 15 minutes [25]. Finally, the supernatant was transferred to the microtube and stored in a freezer at -80°C to measure biochemical indicators of oxidative stress.
Measurement of malondialdehyde (MDA) concentration
To measure malondialdehyde (MDA), 1 mL of the sample was added to 2 mL of a solution containing thiobarbituric acid, trichloroacetic acid, and hydrochloric acid. The mixture was boiled in a warm water bath for 40 min. After reaching room temperature, the solution was centrifuged at 1000 rpm for 10 min. The absorbance of the samples was then read at 532 nm [25].
Measuring the amount of tom thiol groups
Reagent 2-nitrobenzoic acid (DTNB) was used to measure the concentration of tom thiol groups in brain tissues. About 25 μL of this reagent was mixed with 50 μL of Tris, 420 μL of water, and 5 μL of samples. The sufficient volume of the mixture was then poured into the cuvette, and the absorption rate was read at wavelengths of 412 nm. Also, 495 μL of water was used as Blank [26].
Measurement of Superoxide Dismutase (SOD) enzyme activity
Superoxide Dismutase (SOD) activity was measured in brain tissues using a calorimetric method. In this method, the autooxidation of pyrogallol leads to superoxide production. The reduction of tetrazolium molecules by superoxide produces the red substance formazan with an absorption of 560 nm. In the presence of the enzyme SOD, superoxide decomposes into hydrogen peroxide and oxygen, and a decrease in the production of the red formazan complex occurs [27].
Measurement of Catalase (CAT) enzyme activity
The activity of the enzyme catalase in brain tissues was based on the method in which hydrogen peroxide is broken down into water and oxygen by this enzyme. Briefly, a mixture of potassium phosphate, hydrogen peroxide, and samples was prepared. The absorbance was then read at 240 nm. The decrease in absorption indicates a high level of enzyme catalase activity [27].
Statistical analysis
The obtained data were analyzed by SPSSv. 20. After ensuring the normality of behavioral and biochemical data by the Kolmogorov-Smirnov test, 1-way analysis of variance, and Tukey post hoc test was used to analyze and compare groups. P<0.05 was considered to determine the significance and comparison between groups.
3. Results
The effect of ethyl acetate fraction of tanacetum parthenium on seizures induced by PTZ
According to the results, intraperitoneal injection of PTZ-induced generalized clonic and tonic-clonic seizures in mice. Pretreatment with ethyl acetate fraction of Tanacetum parthenium at doses of 50, 100, and 200 mg/kg significantly delayed the onset of clonic seizures in Fraction50 + PTZ, Fraction100 + PTZ, and Fraction200 + PTZ groups compared to the PTZ group (P<0.001). In addition, the delay in the onset of clonic seizures was significantly higher in the group treated with 200 mg/kg compared to the groups treated with doses of 25, 50, and 100 mg (P<0.001) (Figure 1A). The results also showed that injection of all four doses of ethyl acetate fraction caused a significant delay in the onset of generalized tonic-clonic seizures in the groups treated with the fraction compared to the group injected with PTZ alone (P<0.05, P<0.01, and P<0.001). Also, the delay in the onset of generalized tonic-clonic seizures in the Fraction200 + PTZ group was significantly higher compared to other groups treated with the fraction (P<0.001) (Figure 1B).
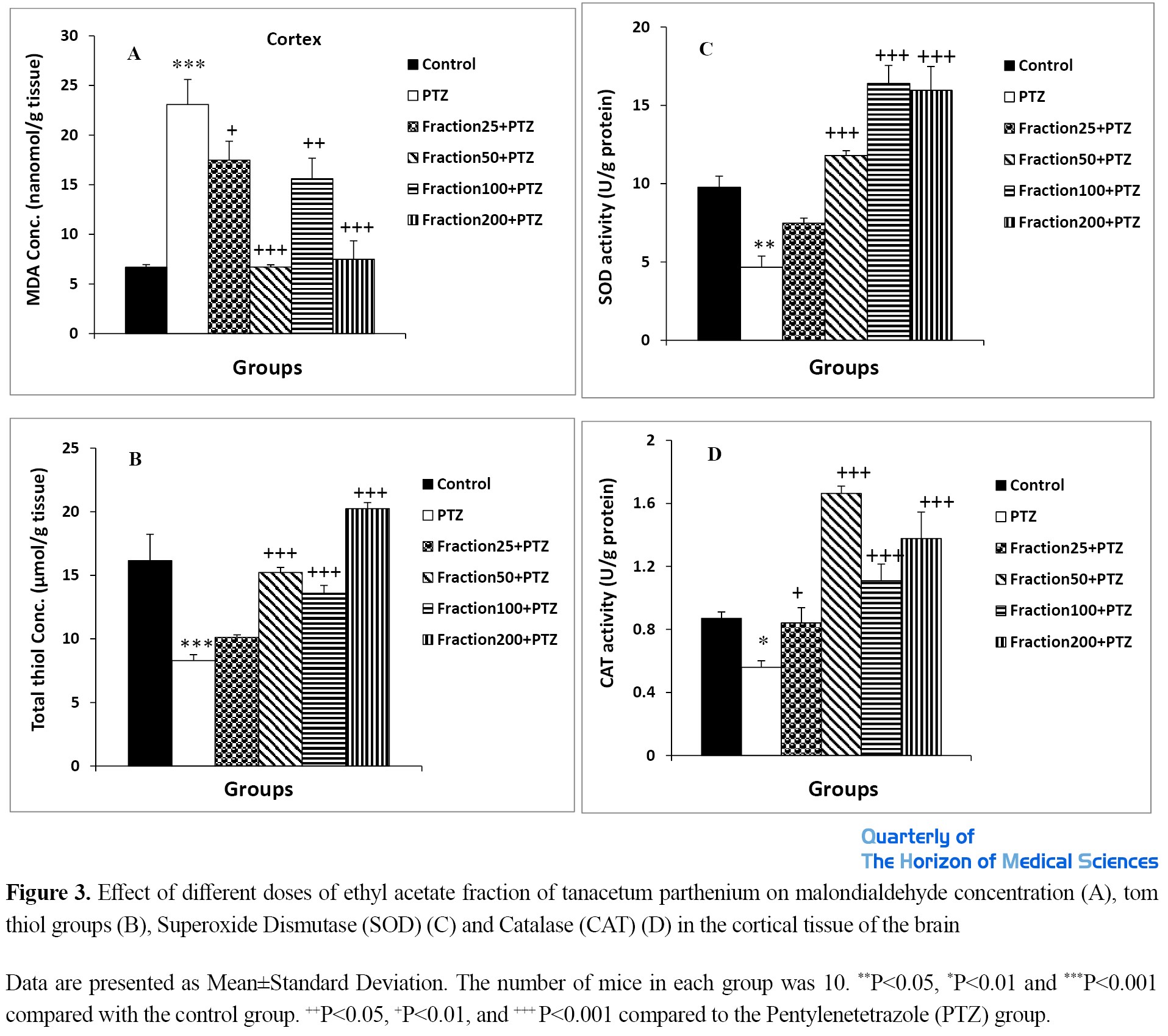
Effect of ethyl acetate fraction of tanacetum parthenium on oxidative stress parameters
As shown in Figures 2-A and 3-A, the amount of malondialdehyde as an indicator of oxidative stress due to lipid peroxidation in hippocampal and cortical tissues in animals treated with PTZ was significantly higher than in the control group (P<0.001). Injection of all four doses of ethyl acetate fraction of Tanacetum parthenium significantly reduced the concentration of this oxidative stress index in Fraction25 + PTZ, Fraction 50 + PTZ, Fraction100 + PTZ, and Fraction200 + PTZ groups compared to PTZ group (P<0.05 to P<0.001).
The results also showed that the level of tom thiol groups in brain tissues in animals of the PTZ group was significantly lower than the control group (P<0.001). Fraction injection resulted in a significant increase in the content of tom thiol groups in hippocampal and cortical tissues in Fraction25 + PTZ, Fraction50 + PTZ, Fraction100 + PTZ, and Fraction200 + PTZ groups compared to the PTZ group (P<0.05 and P<0.01) (Figures 2B and 3B).
Evaluation of the activity of SOD and catalase enzymes in brain tissues also showed that the activity of these anti-oxidant enzymes in the PTZ group was significantly lower than in the control group (P<0.05 and P<0.01). According to the results, injection of plant ethyl acetate fraction significantly increased the activity of these enzymes in hippocampal and cortical tissues in Fraction50 + PTZ, Fraction100 + PTZ, and Fraction200 + PTZ groups compared to the PTZ group (P<0.05 and P<0.01) (Figures 2C & D; 3C and 3D).
4. Discussion
In the current study, intraperitoneal injection of Pentylenetetrazole (PTZ) resulted in severe neuronal depletion with accelerated onset of colonized seizures and generalized tonic-clonic seizures. According to scientific reports, oxidative and nitrous stress plays a significant role in the pathophysiology of seizures [28]. Oxidative and nitrous stress is caused by an imbalance between the production of reactive oxygen and nitrogen species and the reduction of the capacity of the endogenous anti-oxidant system to eliminate free radicals [29]. The brain is very sensitive to oxidative stress and nitrous stress due to its high metabolic activity [30]. Overproduction of reactive oxygen and nitrogen species can increase neuronal excitability and neuronal death through various mechanisms, including damage to neurotransmitter receptors and ion channels [31]. In animal models, seizures induced by kainic acid [32] and PTZ [33] also increase oxidative stress and neuronal death. The current study results also show high concentrations of malondialdehyde, low levels of tom thiol groups, and decreased levels of superoxide dismutase and catalase activity in hippocampal and cortical tissues in the PTZ-treated group compared with the control group. Based on these results, it appears that the high neuronal excitability observed in the PTZ group compared to the control group was due to the induction of oxidative brain damage by PTZ.
Tanacetum parthenium is one of the plants used in traditional medicine that its anti-asthmatic, anti-fever, anti-inflammatory, anti-diabetic and anti-migraine effects have been studied and approved [15, 20]. Scientific evidence has shown that the use of this plant improves the frequency and severity of migraine headaches in children. This effect has been attributed to its reducing serotonin release and attenuating inflammatory responses [34]. In the PTZ-induced epileptic model, delay in the onset of clonic seizures and generalized tonic-clonic seizures are considered important indicators of seizure severity [22]. According to the current study results, pretreatment with ethyl acetate fraction of Tanacetum parthenium reduced the severity of seizures caused by PTZ injection. Delay in the onset of clonic and tonic-clonic seizures in the groups treated with fractions of this plant compared to the group treated with PTZ confirms this finding.
Scientific evidence indicates the critical role of anti-oxidant compounds in cell defense against reactive oxygen and nitrogen species [35, 36]. Researchers have reported that anti-oxidant compounds, including vitamin E, can reduce the production of oxidative stress indicators and weaken seizure frequency [37]. In addition, recent studies have shown that compounds that increase glutathione levels protect the brain against oxidative stress, reduce epileptic seizures, prevent neuronal death, and improve cognitive impairment [31]. Lipid peroxidation is one of the important indicators of oxidative stress, and free radical scavenging is also an important anti-oxidant mechanism in inhibiting lipid peroxidation and malondialdehyde production [38]. The results of studies show that the methanolic extract of Tanacetum parthenium could improve kidney damage caused by carbon tetrachloride by reducing oxidative stress. This effect was accompanied by a decrease in the level of malondialdehyde and an increase in the level of anti-oxidant enzymes from superoxide dismutase and glutathione peroxidase in kidney tissue [15]. Also, the hepatoprotective effect of methanolic extract of this plant has been documented by increasing the levels of glutathione peroxidase and superoxide dismutase enzymes [38]. In addition, in our previous study, the effect of n-butanol and aqueous fractions of Tanacetum parthenium on oxidative damage caused by PTZ-induced seizures was studied. Based on the behavioral results, both fractions could delay the onset of tonic and tonic-clonic seizures. However, based on biochemical results, these two fractions could not improve the oxidative damage caused by an injection of pentylenetetrazole in brain tissue [21]. In the current study, the effect of the ethyl acetate fraction of this plant was investigated. The results showed that, in addition to increasing the delay in the onset of seizures, the ethyl acetate fraction of the plant could also improve the oxidative stress situation caused by PTZ in brain tissue.
According to the results, the level of malondialdehyde in brain tissue decreased while the level of tom thiol groups and the activity of catalase and superoxide dismutase increased. Flavonoids are among the plant compounds that can be separated by ethyl acetate, which have anti-inflammatory and anti-oxidant properties [39]. The researchers reported that the flavonoid-rich ethyl acetate fraction of Acacia hydaspica could protect kidney tissue from oxidative damage caused by the anti-cancer compound doxorubicin [40]. According to these reports, it is possible that in the current study, flavonoid compounds in the ethyl acetate fraction of Tanacetum parthenium plant against oxidative brain damage in PTZ-induced seizures were involved. Still, this issue needs further study in the future. Finally, it is suggested that the effect of the ethyl acetate fraction of Tanacetum parthenium on neuronal death due to seizures induced by PTZ be investigated in future studies. In addition, it is suggested that the effects of parthenolide extracted from the Tanacetum parthenium plant on seizures be studied.
In summary, the ethyl acetate fraction of Tanacetum parthenium, like its n-butanol and aqueous fractions, reduced PTZ-stimulated seizures. According to the results, the ethyl acetate fraction, unlike the other two fractions, improved the state of oxidative stress caused by seizures stimulated by pentylenetetrazole in brain tissue.
Ethical Considerations
Compliance with ethical guidelines
The current research has ethically approved the ethical committee of th Vice Chancellor for Research and Technology of Mashhad University of Medical Sciences
(License number: IR.MUMS.fm.REC.1397.134)
Funding
The research was funded by the Vice-Chancellor for Research and Technology of Mashhad University of Medical Sciences.
Authors' contributions
All authors equally contributed to preparing this article.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors sincerely thank the Vice Chancellor for Research of Mashhad University of Medical Sciences for providing financial facilities for this project.
References
Epilepsy is one of the most common neurological disorders that affect many people worldwide [1]. Epilepsy is characterized by reversible spontaneous seizures caused by an imbalance between irritability and inhibition of the brain with a tendency toward uncontrolled irritability [2]. Epilepsy can be divided into two main types: 1) epilepsy of the middle part of the temporal lobe, which affects the hippocampus, parahippocampus, and amygdala, 2) lateral lobe epilepsy, which affects the neocortex [3]. Experimental models of epilepsy induction were used to study the pathophysiology of epileptic seizures and play an essential role in our better understanding of the molecular mechanisms associated with seizure progression [4]. One of the animal models for studying epileptic seizures is the model in which Pentylenetetrazole (PTZ) is used [5]. Increased production of free radicals plays an important role in the pathogenesis of a wide range of neurological disorders, including epilepsy [6]. The hypersensitivity of the brain to oxidative damage highlights the importance of knowing the role of oxidative stress in the pathogenesis of seizures and epilepsy [7]. Scientific evidence also suggests an association between long-term seizures and oxidative damage to lipids, DNA, and proteins [8]. In addition, neurotoxicity induced by some neurotransmitters, including glutamate, is one of the main mechanisms of neurological seizure disorder [9]. For example, researchers have reported that neurotoxicity due to glutamate overactivity leads to overproduction of nitric oxide by the enzyme nitric oxide synthase, resulting in neuronal damage induced by oxidative stress [10]. Scientific evidence also shows that oxidative damage to biomolecules plays an essential role in neuronal hyperstimulation, neuronal damage, and induction of seizures [11]. The results of recent studies confirm that dysfunction of mitochondria as organs affecting oxidative phosphorylation and acute oxidative stress can predispose the brain to epileptic seizures [12]. Scientific reports also indicate the role of oxidative stress in neuronal damage in a model of epilepsy induced by PTZ [13].
Previous experimental studies show that natural compounds in plant extracts are good sources for relieving seizures and reducing their effects in epileptic patients [14]. Tanacetum parthenium belongs to the Cassian family and is widely grown worldwide [15]. Parthenolide is one of the most important compounds in the extract of this plant with anti-migraine properties [16]. In addition, the extract of this plant is used in traditional medicine to reduce fever, relieve asthma, and reduce inflammation [17]. Scientific results show that the polyphenolic compounds and flavonoids in the extract of Tanacetum parthenium have anti-oxidant properties [18]. The researchers reported that the alcoholic extract of this plant has anti-oxidant activity against diphenyl-2-picrylhydrazyl by neutralizing free radicals [19]. The protective effects of this plant extract against kidney damage caused by carbon tetrachloride by improving the state of oxidative stress have also been documented [15]. Also, the anti-oxidant effects of this plant extract against liver damage caused by excessive production of free radicals along with lowering cholesterol and triglycerides have been shown [20]. The results of our previous study showed that the moderate dose (100 mg/kg) of hydroalcoholic extract of Tanacetum parthenium had the best effect against PTZ-induced seizures in mice by decreasing malondialdehyde levels and increasing levels of tom thiols and the anti-oxidant enzymes superoxide dismutase and catalase. In contrast, the n-butanol and aqueous fractions could not improve the oxidative brain damage caused by PTZ-induced seizures [21]. Therefore, compounds other than soluble compounds in n-butanol and aqueous solvents of this plant may have a role in reducing oxidative brain damage caused by pentylenetetrazole-induced seizures. Therefore, to clarify the issue in this study, the effects of ethyl acetate fraction of Tanacetum parthenium plant against oxidative brain damage in a model of PTZ-induced seizures in rats were investigated.
2. Materials and Methods
Preparation of Ethyl Acetate Fraction
To prepare the ethyl acetate fraction, 50 g of plant powder was dissolved in 300 mL of ethanol and extracted using an extractor. Next, 10 g of dried ethanolic extract was dissolved in 50 mL of distilled water and poured into a decanter. Then 50 mL of n-butanol solvent was added to it. In this stage, two phases are formed; the upper phase contains soluble compounds in butanol. After separating the two phases from each other, to prepare the ethyl acetate fraction, the lower phase of the previous step was poured into the decanter, and 50 mL of ethyl acetate solvent was added to it. Two phases are formed again, the upper phase containing ethyl acetate-soluble compounds. After separation, the resulting solution was dried by an incubator and stored for use [21].
Animals and Groups
This study is an experimental study in which 60 male mice weighing 30±5 g were randomly divided into 6 groups of 10 as follows: 1) control group, 2) pentylenetetrazole (PTZ) group, 3) PTZ + fraction with a dose of 25 mg/kg (Fraction25 + PTZ), 4) PTZ + fraction with a dose of 50 mg/kg (Fraction50 + PTZ), 5) PTZ + fraction with a dose of 100 mg/kg (Fraction100 + PTZ), and 6) PTZ + fraction at a dose of 200 mg/kg (Fraction200 + PTZ). The ethyl acetate fraction was injected intraperitoneally for three weeks 30 minutes before pentylenetetrazole. Animals in the control group also received saline. The animals were kept under standard conditions (temperature 22°C-24°C, light period 12 hours of light and 12 hours of darkness, humidity 50%, and adequate access to water and food). The dose of drugs in this study was selected based on previous studies [21]. Before the beginning of the experiments, the proposal of this plan was presented in the Ethics Committee of Mashhad University of Medical Sciences. The ethical code (IR.MUMS.fm.REC.1397.134) belongs to the Ethics Committee of Mashhad University of Medical Sciences.
Induction of seizures by pentylenetetrazole
To induce seizures in mice, pentylenetetrazole (100 mg/kg body weight) was injected intraperitoneally [22, 23]. The animals were then placed in a Plexiglas box, and their convulsive behaviors were assessed for 60 min based on the Becker 5-point method: stage 0, no response; stage 1, face and ear muscle impulses; stage 2, myoclonic impulses without standing on two legs; stage 3, myoclonic impulses with standing on two legs; stage 4, falling to one side and tonic-clonic seizures; and stage 5, falling on the back and generalized tonic-clonic seizures. If stages 4 and 5 were observed, the animal was considered convulsive [24]. Acceleration of the onset of clonic seizures (minimal clonic seizure [MCS]) and generalized tonic-clonic seizures (GTCS) were considered the criteria of seizure severity. After behavioral experiments, the animals were killed, their brains were removed, and the hippocampal and cortical tissues were isolated. Brain tissues were homogenized in 1 mL of phosphate buffer. The homogenized tissue was then centrifuged at 3000 rpm for 15 minutes [25]. Finally, the supernatant was transferred to the microtube and stored in a freezer at -80°C to measure biochemical indicators of oxidative stress.
Measurement of malondialdehyde (MDA) concentration
To measure malondialdehyde (MDA), 1 mL of the sample was added to 2 mL of a solution containing thiobarbituric acid, trichloroacetic acid, and hydrochloric acid. The mixture was boiled in a warm water bath for 40 min. After reaching room temperature, the solution was centrifuged at 1000 rpm for 10 min. The absorbance of the samples was then read at 532 nm [25].
Measuring the amount of tom thiol groups
Reagent 2-nitrobenzoic acid (DTNB) was used to measure the concentration of tom thiol groups in brain tissues. About 25 μL of this reagent was mixed with 50 μL of Tris, 420 μL of water, and 5 μL of samples. The sufficient volume of the mixture was then poured into the cuvette, and the absorption rate was read at wavelengths of 412 nm. Also, 495 μL of water was used as Blank [26].
Measurement of Superoxide Dismutase (SOD) enzyme activity
Superoxide Dismutase (SOD) activity was measured in brain tissues using a calorimetric method. In this method, the autooxidation of pyrogallol leads to superoxide production. The reduction of tetrazolium molecules by superoxide produces the red substance formazan with an absorption of 560 nm. In the presence of the enzyme SOD, superoxide decomposes into hydrogen peroxide and oxygen, and a decrease in the production of the red formazan complex occurs [27].
Measurement of Catalase (CAT) enzyme activity
The activity of the enzyme catalase in brain tissues was based on the method in which hydrogen peroxide is broken down into water and oxygen by this enzyme. Briefly, a mixture of potassium phosphate, hydrogen peroxide, and samples was prepared. The absorbance was then read at 240 nm. The decrease in absorption indicates a high level of enzyme catalase activity [27].
Statistical analysis
The obtained data were analyzed by SPSSv. 20. After ensuring the normality of behavioral and biochemical data by the Kolmogorov-Smirnov test, 1-way analysis of variance, and Tukey post hoc test was used to analyze and compare groups. P<0.05 was considered to determine the significance and comparison between groups.
3. Results
The effect of ethyl acetate fraction of tanacetum parthenium on seizures induced by PTZ
According to the results, intraperitoneal injection of PTZ-induced generalized clonic and tonic-clonic seizures in mice. Pretreatment with ethyl acetate fraction of Tanacetum parthenium at doses of 50, 100, and 200 mg/kg significantly delayed the onset of clonic seizures in Fraction50 + PTZ, Fraction100 + PTZ, and Fraction200 + PTZ groups compared to the PTZ group (P<0.001). In addition, the delay in the onset of clonic seizures was significantly higher in the group treated with 200 mg/kg compared to the groups treated with doses of 25, 50, and 100 mg (P<0.001) (Figure 1A). The results also showed that injection of all four doses of ethyl acetate fraction caused a significant delay in the onset of generalized tonic-clonic seizures in the groups treated with the fraction compared to the group injected with PTZ alone (P<0.05, P<0.01, and P<0.001). Also, the delay in the onset of generalized tonic-clonic seizures in the Fraction200 + PTZ group was significantly higher compared to other groups treated with the fraction (P<0.001) (Figure 1B).
Effect of ethyl acetate fraction of tanacetum parthenium on oxidative stress parameters
As shown in Figures 2-A and 3-A, the amount of malondialdehyde as an indicator of oxidative stress due to lipid peroxidation in hippocampal and cortical tissues in animals treated with PTZ was significantly higher than in the control group (P<0.001). Injection of all four doses of ethyl acetate fraction of Tanacetum parthenium significantly reduced the concentration of this oxidative stress index in Fraction25 + PTZ, Fraction 50 + PTZ, Fraction100 + PTZ, and Fraction200 + PTZ groups compared to PTZ group (P<0.05 to P<0.001).
The results also showed that the level of tom thiol groups in brain tissues in animals of the PTZ group was significantly lower than the control group (P<0.001). Fraction injection resulted in a significant increase in the content of tom thiol groups in hippocampal and cortical tissues in Fraction25 + PTZ, Fraction50 + PTZ, Fraction100 + PTZ, and Fraction200 + PTZ groups compared to the PTZ group (P<0.05 and P<0.01) (Figures 2B and 3B).
Evaluation of the activity of SOD and catalase enzymes in brain tissues also showed that the activity of these anti-oxidant enzymes in the PTZ group was significantly lower than in the control group (P<0.05 and P<0.01). According to the results, injection of plant ethyl acetate fraction significantly increased the activity of these enzymes in hippocampal and cortical tissues in Fraction50 + PTZ, Fraction100 + PTZ, and Fraction200 + PTZ groups compared to the PTZ group (P<0.05 and P<0.01) (Figures 2C & D; 3C and 3D).
4. Discussion
In the current study, intraperitoneal injection of Pentylenetetrazole (PTZ) resulted in severe neuronal depletion with accelerated onset of colonized seizures and generalized tonic-clonic seizures. According to scientific reports, oxidative and nitrous stress plays a significant role in the pathophysiology of seizures [28]. Oxidative and nitrous stress is caused by an imbalance between the production of reactive oxygen and nitrogen species and the reduction of the capacity of the endogenous anti-oxidant system to eliminate free radicals [29]. The brain is very sensitive to oxidative stress and nitrous stress due to its high metabolic activity [30]. Overproduction of reactive oxygen and nitrogen species can increase neuronal excitability and neuronal death through various mechanisms, including damage to neurotransmitter receptors and ion channels [31]. In animal models, seizures induced by kainic acid [32] and PTZ [33] also increase oxidative stress and neuronal death. The current study results also show high concentrations of malondialdehyde, low levels of tom thiol groups, and decreased levels of superoxide dismutase and catalase activity in hippocampal and cortical tissues in the PTZ-treated group compared with the control group. Based on these results, it appears that the high neuronal excitability observed in the PTZ group compared to the control group was due to the induction of oxidative brain damage by PTZ.
Tanacetum parthenium is one of the plants used in traditional medicine that its anti-asthmatic, anti-fever, anti-inflammatory, anti-diabetic and anti-migraine effects have been studied and approved [15, 20]. Scientific evidence has shown that the use of this plant improves the frequency and severity of migraine headaches in children. This effect has been attributed to its reducing serotonin release and attenuating inflammatory responses [34]. In the PTZ-induced epileptic model, delay in the onset of clonic seizures and generalized tonic-clonic seizures are considered important indicators of seizure severity [22]. According to the current study results, pretreatment with ethyl acetate fraction of Tanacetum parthenium reduced the severity of seizures caused by PTZ injection. Delay in the onset of clonic and tonic-clonic seizures in the groups treated with fractions of this plant compared to the group treated with PTZ confirms this finding.
Scientific evidence indicates the critical role of anti-oxidant compounds in cell defense against reactive oxygen and nitrogen species [35, 36]. Researchers have reported that anti-oxidant compounds, including vitamin E, can reduce the production of oxidative stress indicators and weaken seizure frequency [37]. In addition, recent studies have shown that compounds that increase glutathione levels protect the brain against oxidative stress, reduce epileptic seizures, prevent neuronal death, and improve cognitive impairment [31]. Lipid peroxidation is one of the important indicators of oxidative stress, and free radical scavenging is also an important anti-oxidant mechanism in inhibiting lipid peroxidation and malondialdehyde production [38]. The results of studies show that the methanolic extract of Tanacetum parthenium could improve kidney damage caused by carbon tetrachloride by reducing oxidative stress. This effect was accompanied by a decrease in the level of malondialdehyde and an increase in the level of anti-oxidant enzymes from superoxide dismutase and glutathione peroxidase in kidney tissue [15]. Also, the hepatoprotective effect of methanolic extract of this plant has been documented by increasing the levels of glutathione peroxidase and superoxide dismutase enzymes [38]. In addition, in our previous study, the effect of n-butanol and aqueous fractions of Tanacetum parthenium on oxidative damage caused by PTZ-induced seizures was studied. Based on the behavioral results, both fractions could delay the onset of tonic and tonic-clonic seizures. However, based on biochemical results, these two fractions could not improve the oxidative damage caused by an injection of pentylenetetrazole in brain tissue [21]. In the current study, the effect of the ethyl acetate fraction of this plant was investigated. The results showed that, in addition to increasing the delay in the onset of seizures, the ethyl acetate fraction of the plant could also improve the oxidative stress situation caused by PTZ in brain tissue.
According to the results, the level of malondialdehyde in brain tissue decreased while the level of tom thiol groups and the activity of catalase and superoxide dismutase increased. Flavonoids are among the plant compounds that can be separated by ethyl acetate, which have anti-inflammatory and anti-oxidant properties [39]. The researchers reported that the flavonoid-rich ethyl acetate fraction of Acacia hydaspica could protect kidney tissue from oxidative damage caused by the anti-cancer compound doxorubicin [40]. According to these reports, it is possible that in the current study, flavonoid compounds in the ethyl acetate fraction of Tanacetum parthenium plant against oxidative brain damage in PTZ-induced seizures were involved. Still, this issue needs further study in the future. Finally, it is suggested that the effect of the ethyl acetate fraction of Tanacetum parthenium on neuronal death due to seizures induced by PTZ be investigated in future studies. In addition, it is suggested that the effects of parthenolide extracted from the Tanacetum parthenium plant on seizures be studied.
In summary, the ethyl acetate fraction of Tanacetum parthenium, like its n-butanol and aqueous fractions, reduced PTZ-stimulated seizures. According to the results, the ethyl acetate fraction, unlike the other two fractions, improved the state of oxidative stress caused by seizures stimulated by pentylenetetrazole in brain tissue.
Ethical Considerations
Compliance with ethical guidelines
The current research has ethically approved the ethical committee of th Vice Chancellor for Research and Technology of Mashhad University of Medical Sciences
(License number: IR.MUMS.fm.REC.1397.134)
Funding
The research was funded by the Vice-Chancellor for Research and Technology of Mashhad University of Medical Sciences.
Authors' contributions
All authors equally contributed to preparing this article.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors sincerely thank the Vice Chancellor for Research of Mashhad University of Medical Sciences for providing financial facilities for this project.
References
- Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia. 2010; 51(6):1069-77. [DOI:10.1111/j.1528-1167.2009.02397.x] [PMID]
- Juhász C, Mittal S. Molecular imaging of brain tumor-associated epilepsy. Diagnostics. 2020; 10(12):1049. [DOI:10.3390/diagnostics10121049] [PMID] [PMCID]
- Waldbaum S, Patel M. Mitochondria, oxidative stress, and temporal lobe epilepsy. Epilepsy Research. 2010; 88(1):23-45. [DOI:10.1016/j.eplepsyres.2009.09.020] [PMID] [PMCID]
- Cardenas-Rodriguez N, Huerta-Gertrudis B, Rivera-Espinosa L, Montesinos-Correa H, Bandala C, Carmona-Aparicio L, et al. Role of oxidative stress in refractory epilepsy: Evidence in patients and experimental models. International Journal of Molecular Sciences. 2013; 14(1):1455-76. [DOI:10.3390/ijms14011455] [PMID] [PMCID]
- Homayoun M, Shafieian R, Seghatoleslam M, Hosseini M, Ebrahimzadeh-Bideskan A. [Protective impact of rosa damascena against neural damage in a rat model of Pentylenetetrazole (PTZ)-induced seizure (Persian)]. Avicenna Journal of Phytomedicine. 2020; 10(6):574-83. [PMID] [PMCID]
- Devi PU, Manocha A, Vohora D. Seizures, antiepileptics, antioxidants and oxidative stress: An insight for researchers. Expert Opinion on Pharmacotherapy. 2008; 9(18):3169-77. [DOI:10.1517/14656560802568230] [PMID]
- Mahmoudi T, Lorigooini Z, Rafieian-Kopaei M, Arabi M, Rabiei Z, Bijad E, et al. [Effect of curcuma zedoaria hydro-alcoholic extract on learning, memory deficits and oxidative damage of brain tissue following seizures induced by pentylenetetrazole in rat (Persian)]. Behavioral and Brain Functions. 2020; 16(1):1-12. [DOI:10.1186/s12993-020-00169-3] [PMID] [PMCID]
- Kudin AP, Kudina TA, Seyfried J, Vielhaber S, Beck H, Elger CE, et al. Seizure-dependent modulation of mitochondrial oxidative phosphorylation in rat hippocampus. European Journal of Neuroscience. 2002; 15(7):1105-14. [DOI:10.1046/j.1460-9568.2002.01947.x] [PMID]
- Lee SJ. Effects of preconditioning exercise on nitric oxide and antioxidants in hippocampus of epileptic seizure. Journal of Exercise Rehabilitation. 2019; 15(6):757. [DOI:10.12965/jer.1938698.349] [PMID] [PMCID]
- Chen HJ, Spiers JG, Sernia C, Anderson ST, Lavidis NA. Reactive nitrogen species contribute to the rapid onset of redox changes induced by acute immobilization stress in rats. Stress. 2014; 17(6):520-7. [DOI:10.3109/10253890.2014.966264] [PMID]
- Kunz WS, Kudin AP, Vielhaber S, Blümcke I, Zuschratter W, Schramm J, et al. Mitochondrial complex I deficiency in the epileptic focus of patients with temporal lobe epilepsy. Annals of Neurology. 2000; 48(5):766-73. [PMID]
- Chuang YC. Mitochondrial dysfunction and oxidative stress in seizure-induced neuronal cell death. Acta Neurologica Taiwanica. 2010; 19(1):3-15. [PMID]
- Obay BD, Taşdemir E, Tümer C, Bilgin H, Atmaca M. Dose dependent effects of ghrelin on pentylenetetrazole-induced oxidative stress in a rat seizure model. Peptides. 2008; 29(3):448-55. [DOI:10.1016/j.peptides.2007.11.020] [PMID]
- Sahranavard S, Ghafari S, Mosaddegh M. [Medicinal plants used in Iranian traditional medicine to treat epilepsy (Persian)]. Seizure. 2014; 23(5):328-32. [DOI:10.1016/j.seizure.2014.01.013] [PMID]
- Mazani M, Mahmoodzadeh Y, Chinifroush Asl MM, Banaei S, Rezagholizadeh L, Mohammadnia A. [Renoprotective effects of the methanolic extract of tanacetum parthenium against carbon tetrachloride-induced renal injury in rats (Persian)]. Avicenna Journal of Phytomedicine. 2018; 8(4):370-79. [PMID] [PMCID]
- Williams CA, Harborne JB, Geiger H, Hoult JR. The flavonoids of tanacetum parthenium and T. Vulgare and their anti-inflammatory properties. Phytochemistry. 1999; 51(3):417-23. [DOI:10.1016/s0031-9422(99)00021-7] [PMID]
- Sharopov FS, Setzer WN, Isupov SJ, Wink M. Composition and bioactivity of the essential oil of tanacetum parthenium from a wild population growing in Tajikistan. American Journal of Essential Oils and Natural Products. 2015; 2(4):32-4. https://www.essencejournal.com/archives/2015/2/4/A/2-4-9
- Pedrielli P, Skibsted LH. Antioxidant synergy and regeneration effect of quercetin, (-)-epicatechin, and (+)-catechin on alpha-tocopherol in homogeneous solutions of peroxidating methyl linoleate. Journal of Agricultural and Food Chemistry. 2002; 50(24):7138-44. [DOI:10.1021/jf020437l] [PMID]
- Wu C, Chen F, Wang X, Kim HJ, He GQ, Haley-Zitlin V, et al. Antioxidant constituents in feverfew (tanacetum parthenium) extract and their chromatographic quantification. Food Chemistry. 2006; 96(2):220-7. [DOI:10.1016/j.foodchem.2005.02.024]
- Mahmoodzadeh Y, Mazani M, Rezagholizadeh L. [Hepatoprotective effect of methanolic tanacetum parthenium extract on CCl4-induced liver damage in rats (Persian)]. Toxicology Reports. 2017; 4:455-62. [DOI:10.1016/j.toxrep.2017.08.003] [PMID] [PMCID]
- Asgharzadeh F, Hosseini M, Bargi R, Beheshti F, Rakhshandeh H, Mansouri S, et al. [Effects of hydro-ethanolic extract of tanacetum parthenium and its N-Butanol and aqueous fractions on brain oxidative damage in pentylenetetrazole-induced seizures in mice (Persian)]. Pharmaceutical Sciences. 2020; 26(3):252-60. [DOI:10.34172/PS.2020.32]
- Asgharzadeh F, Hosseini M, Bargi R, Soukhtanloo M, Beheshti F, Mohammady Z, et al. [Effect of captopril on brain oxidative damage in pentylenetetrazole-induced seizures in mice (Persian)]. Pharmaceutical Sciences. 2019; 25(3):221-6. [DOI:10.15171/PS.2019.38]
- Pourzaki M, Homayoun M, Sadeghi S, Seghatoleslam M, Hosseini M, Ebrahimzadeh Bideskan A. [Preventive effect of coriandrum sativum on neuronal damages in pentylentetrazole-induced seizure in rats (Persian)]. Avicenna Journal of Phytomedicine. 2017; 7(2):116-28. [PMID] [PMCID]
- Pourmotabbed A, Nedaei SE, Cheraghi M, Moradian S, Touhidi A, Aeinfar M, et al. [Effect of prenatal pentylenetetrazol-induced kindling on learning and memory of male offspring (Persian)]. Neuroscience. 2011; 172:205-11. [DOI:10.1016/j.neuroscience.2010.11.001] [PMID]
- Asgharzadeh F, Bargi R, Hosseini M, Farzadnia M, Khazaei M. [Cardiac and renal fibrosis and oxidative stress balance in lipopolysaccharide-induced inflammation in male rats (Persian)]. ARYA Atheroscler. 2018; 14(2):71-77. [DOI:10.22122/arya.v14i2.1550] [PMID] [PMCID]
- Asgharzadeh F, Bargi R, Beheshti F, Hosseini M, Farzadnia M, Khazaei M. [Thymoquinone prevents myocardial and perivascular fibrosis induced by chronic lipopolysaccharide exposure in male rats: Thymoquinone and cardiac fibrosis (Persian)]. Journal of Pharmacopuncture. 2018; 21(4):284-93. [DOI:10.3831/KPI.2018.21.032] [PMID] [PMCID]
- Beheshti F, Hosseini M, Hashemzehi M, Soukhtanloo M, Khazaei M, Shafei MN. [The effects of PPAR-γ agonist pioglitazone on hippocampal cytokines, brain-derived neurotrophic factor, memory impairment, and oxidative stress status in lipopolysaccharide-treated rats (Persian)]. Iranian Journal of Basic Medical Sciences. 2019; 22(8):940-48. [DOI:10.22038/ijbms.2019.36165.8616] [PMID] [PMCID]
- Aguiar CC, Almeida AB, Araújo PV, de Abreu RN, Chaves EM, do Vale OC, et al. Oxidative stress and epilepsy: literature review. Oxidative Medicine and Cellular Longevity. 2012; 2012:795259. [DOI:10.1155/2012/795259] [PMID] [PMCID]
- Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant physiology. 2006; 141(2):312-22. [DOI:10.1104/pp.106.077073] [PMID] [PMCID]
- Salim S. Oxidative stress and the central nervous system. Journal of Pharmacology and Experimental Therapeutics. 2017; 360(1):201-5. [DOI:10.1124/jpet.116.237503] [PMID] [PMCID]
- Pearson-Smith JN, Patel M. Metabolic dysfunction and oxidative stress in epilepsy. International Journal of Molecular Sciences. 2017; 18(11):2365. [DOI:10.3390/ijms18112365] [PMID] [PMCID]
- Gupta YK, Briyal S, Chaudhary G. Protective effect of trans-resveratrol against kainic acid-induced seizures and oxidative stress in rats. Pharmacology Biochemistry and Behavior. 2002; 71(1-2):245-9. [DOI:10.1016/s0091-3057(01)00663-3] [PMID]
- Erbas O, Yılmaz M, Korkmaz HA, Bora S, Evren V, Peker G. Oxytocin inhibits pentylentetrazol-induced seizures in the rat. Peptides. 2013; 40:141-4. [DOI:10.1016/j.peptides.2012.12.003] [PMID]
- Moscano F, Guiducci M, Maltoni L, Striano P, Ledda MG, Zoroddu F, et al. An observational study of fixed-dose tanacetum parthenium nutraceutical preparation for prophylaxis of pediatric headache. Italian Journal of Pediatrics. 2019; 45(1):36. [DOI:10.1186/s13052-019-0624-z] [PMID] [PMCID]
- Urquiaga I, Leighton F. Plant polyphenol antioxidants and oxidative stress. Biological Research. 2000; 33(2):55-64. [DOI:10.4067/s0716-97602000000200004] [PMID]
- Yuan X, Fu Z, Ji P, Guo L, Al-Ghamdy AO, Alkandiri A, et al. Selenium nanoparticles pre-treatment reverse behavioral, oxidative damage, neuronal loss and neurochemical alterations in pentylenetetrazole-induced epileptic seizures in mice. International Journal of Nanomedicine. 2020; 15:6339-353. [DOI:10.2147/IJN.S259134] [PMID] [PMCID]
- Mehvari J, Motlagh FG, Najafi M, Ghazvini MR, Naeini AA, Zare M. [Effects of vitamin E on seizure frequency, electroencephalogram findings, and oxidative stress status of refractory epileptic patients (Persian)]. Advanced Biomedical Research. 2016; 5:36. [DOI:10.4103/2277-9175.178780] [PMID] [PMCID]
- Vuda M, D’Souza R, Upadhya S, Kumar V, Rao N, Kumar V, et al. Hepatoprotective and antioxidant activity of aqueous extract of hybanthus enneaspermus against CCl4-induced liver injury in rats. Experimental and Toxicologic Pathology. 2012; 64(7-8):855-9. [DOI:10.1016/j.etp.2011.03.006] [PMID]
- Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: A review of probable mechanisms of action and potential applications. American Journal of Clinical Nutrition. 2001; 74(4):418-25. [DOI:10.1093/ajcn/74.4.418] [PMID]
- Afsar T, Razak S, Almajwal A, Al-Disi D. [Doxorubicin-induced alterations in kidney functioning, oxidative stress, DNA damage, and renal tissue morphology; Improvement by acacia hydaspica tannin-rich ethyl acetate fraction (Persian)]. Saudi Journal of Biological Sciences. 2020; 27(9):2251-2260. [DOI:10.1016/j.sjbs.2020.07.011] [PMID] [PMCID]
Type of Study: Original |
Subject:
Physiology
Received: 2020/10/29 | Accepted: 2021/02/2 | Published: 2021/09/23
Received: 2020/10/29 | Accepted: 2021/02/2 | Published: 2021/09/23
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



.jpg)
.jpg)