Volume 27, Issue 2 (Spring 2021)
Intern Med Today 2021, 27(2): 214-229 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sanjari R, Hosseini S E. Effects of Perinatal and Neonatal Sodium Nitrite on Serum Levels of Uric Acid, Urea, Creatinine, and Tissue Structure of Rats’ Offspring Kidneys. Intern Med Today 2021; 27 (2) :214-229
URL: http://imtj.gmu.ac.ir/article-1-3509-en.html
URL: http://imtj.gmu.ac.ir/article-1-3509-en.html
1- Department of Biology, Faculty of Science, Shiraz Branch, Islamic Azad University, Shiraz, Iran.
2- Department of Biology, Faculty of Science, Zand Institute of Higher Education, Shiraz, Iran. , ebrahhim.hossini@yahoo.cm
2- Department of Biology, Faculty of Science, Zand Institute of Higher Education, Shiraz, Iran. , ebrahhim.hossini@yahoo.cm
Full-Text [PDF 4708 kb]
(905 Downloads)
| Abstract (HTML) (1541 Views)
Full-Text: (1387 Views)
1. Introduction
Semi-finished meat products that are stored in the refrigerator have a limited shelf life [1]. Food products are usually spoiled by chemical changes or increased microbial load [2]. The presence of high concentrations of nitrate in aquatic environments, especially drinking water, leads to short-term and long-term adverse effects in the body and can cause diseases such as methemoglobinemia, cancer, and fetal disorders [3]. In addition to the effect of fat oxidation in reducing the quality of meat products, spoilage due to microbial contamination can create severe health risks to consumers. Accordingly, it seems that the use of appropriate substances with antibacterial and antioxidant activity is valuable and necessary to improve quality, increase durability, and at the same time prevent economic losses [4, 5]. Nitrate and nitrite compounds are mainly used to stabilize the color of lean meat tissues, to flavor processed meat products, and to prevent the growth of spoilage microorganisms and subsequent food poisoning [6]. Nitrates and nitrites are the critical additives in producing processed meat products that can damage blood vessels, liver, spleen, and other organs [7]. Despite the desirable properties of nitrites, they can react with amines and free amino acids in meat products under certain conditions and produce nitrogen amines [8]. Human contact with nitrate and nitrite compounds is mainly due to food consumption, especially vegetables, meat, and contaminated water [9]. When the pH of the stomach is acidic and intestinal bacteria are present in the intestine, nitrite compounds react quickly with secondary amines and amides, resulting in the production of the carcinogenic compounds N-nitroso [10].
Excessive use of food additives has greatly exposed humans to preservatives such as sodium nitrite [11]. Nitrites are at the center of oxidation and reduction reactions in the body, which can be oxidized to the highly biologically active radical nitric oxide and mainly to the nitrate anion [12]. Peroxynitrite (O-ONO) derived from compounds containing nitrite or nitrate can easily cross the phospholipid membranes of various cells and reacts with many molecules such as lipids, proteins, and DNA, and cause cell death by necrosis or induction of apoptosis [13]. Peroxynitrite and nitric oxide affect the cardiovascular system by different mechanisms and cause cell death and various tissue damages [14]. Sodium nitrite in different doses can reduce the thickness of the middle layer of the arteries by increasing the amount of nitric oxide in the blood and cause a variety of other disorders [15]. Consumption of sodium nitrite in drinking water in male and female rats causes hepatocellular degeneration and necrosis and hemosiderin deposition in the liver, spleen, and lymph nodes and hemolysis [16]. Nitrites and nitrates are both precursors of NO (Nitric Oxide) radicals that quickly pass through two phospholipid layers of the membrane by creating oxidative stress and producing ONOO- and reacting with many target molecules such as lipids, proteins, and DNA, and ultimately cell death through necrosis or apoptosis [17]. Nitrite-containing diets have been shown to adversely affect the mechanism of membrane cell proliferation within the vessels [18].
Most studies done on preservatives such as sodium nitrite have examined their effects on the tissue and functional structures of the consumers. So far, few studies (at least with the conditions governing this study) have been conducted in connection with the effects of perinatal and neonatal consumption of these compounds on the tissue and functional structure of children’s kidneys. So the study of the effect of such substances on fetuses and infants is of particular importance. This study aimed to investigate the perinatal and neonatal effects of sodium nitrite on the tissue and functional structure of the kidneys of rats’ offspring.
Excessive use of food additives has greatly exposed humans to preservatives such as sodium nitrite [11]. Nitrites are at the center of oxidation and reduction reactions in the body, which can be oxidized to the highly biologically active radical nitric oxide and mainly to the nitrate anion [12]. Peroxynitrite (O-ONO) derived from compounds containing nitrite or nitrate can easily cross the phospholipid membranes of various cells and reacts with many molecules such as lipids, proteins, and DNA, and cause cell death by necrosis or induction of apoptosis [13]. Peroxynitrite and nitric oxide affect the cardiovascular system by different mechanisms and cause cell death and various tissue damages [14]. Sodium nitrite in different doses can reduce the thickness of the middle layer of the arteries by increasing the amount of nitric oxide in the blood and cause a variety of other disorders [15]. Consumption of sodium nitrite in drinking water in male and female rats causes hepatocellular degeneration and necrosis and hemosiderin deposition in the liver, spleen, and lymph nodes and hemolysis [16]. Nitrites and nitrates are both precursors of NO (Nitric Oxide) radicals that quickly pass through two phospholipid layers of the membrane by creating oxidative stress and producing ONOO- and reacting with many target molecules such as lipids, proteins, and DNA, and ultimately cell death through necrosis or apoptosis [17]. Nitrite-containing diets have been shown to adversely affect the mechanism of membrane cell proliferation within the vessels [18].
Most studies done on preservatives such as sodium nitrite have examined their effects on the tissue and functional structures of the consumers. So far, few studies (at least with the conditions governing this study) have been conducted in connection with the effects of perinatal and neonatal consumption of these compounds on the tissue and functional structure of children’s kidneys. So the study of the effect of such substances on fetuses and infants is of particular importance. This study aimed to investigate the perinatal and neonatal effects of sodium nitrite on the tissue and functional structure of the kidneys of rats’ offspring.
2. Materials and Methods
This experimental study was performed on 56 female Wistar rats in Islamic Azad University, Shiraz Branch, Shiraz City, Iran, in 2018. In this study, rats were divided into seven groups of 8: control group (untreated), perinatal and neonatal controls (treated with solvent), and experimental perinatal groups (treated with doses of 90 and 180 mg/kg sodium nitrite during pregnancy) and neonates (treated with doses of 90 and 180 mg/kg sodium nitrites during lactation). In this study, all prescriptions were performed by gavage. Eight adult male rats were used to conceive rats. This research protocol is based on the international law on laboratory animals and was approved by the University Ethics Committee IR.miau1395 1016. The rats of prenatal groups from the first day of pregnancy to the end of the period and rats of neonatal groups from the first day of birth to the end of lactation were prescribed sodium nitrite. At the end of lactation, 8 male offspring were randomly selected from each group, and after anesthesia with ketamine, their blood samples were taken from their hearts, and sufficient serum was prepared to measure urea, creatinine, and uric acid factors. Then, by separating their kidney organs and fixing them with 10% formalin for one week, tissue sections were prepared with the help of a tissue processor and a fully automatic microtome. Tissue sections were prepared, stained with hematoxylin-eosin, and then evaluated.
In this study, creatinine was measured by Jaffe/Fixed Rate or Kinetic method, and urea and uric acid were measured by Berthelot/Endpoint method using appropriate kits (made by ParsAzmoun Co., Iran). Finally, the data of this study were analyzed using ANOVA and Tukey statistical tests in SPSS v. 22. The significance of the data difference was considered at the level of P<0.05.
In this study, creatinine was measured by Jaffe/Fixed Rate or Kinetic method, and urea and uric acid were measured by Berthelot/Endpoint method using appropriate kits (made by ParsAzmoun Co., Iran). Finally, the data of this study were analyzed using ANOVA and Tukey statistical tests in SPSS v. 22. The significance of the data difference was considered at the level of P<0.05.
3. Results
The results showed that sodium nitrite consumption at doses of 90 and 180 mg/kg during pregnancy (prenatal) in first-generation infants caused a significant increase in their serum urea, creatinine, and uric acid compared to the control group (P<0.001) (Tables 1 and 2).
.jpg)
.jpg)
The results also showed that sodium nitrite consumption at doses of 90 and 180 mg/kg during lactation (prenatal) in rats’ offspring caused a significant increase in their serum urea (P<0.003), creatinine, and uric acid compared to the control group (P<0.001) (Tables 3 and 4).
.jpg)

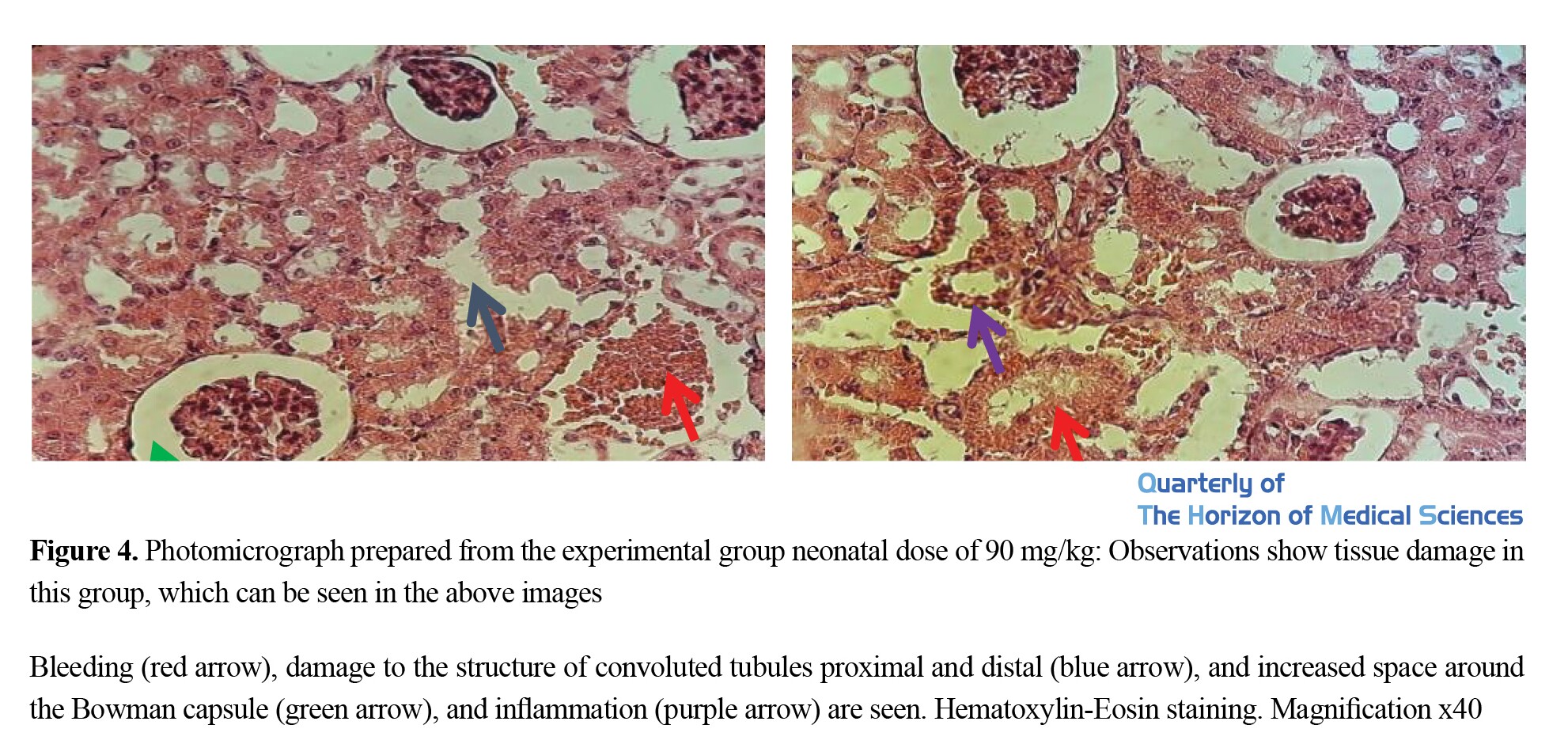
Also, the results of histological studies showed that sodium nitrite consumption during pregnancy and lactation causes a lot of damage to the structure of rats’ offspring renal tissues, such as congestion and diffuse bleeding, damage to the structure of proximal and distal convoluted tubules, dilation, and increase the space around the Bowman capsule, glomerular atrophy, and inflammation around the tubules as well as tubular damage (Figures 1, 2, 3, 4, & 5).
.jpg)
.jpg)
.jpg)
.jpg)
The results also showed that sodium nitrite consumption at doses of 90 and 180 mg/kg during lactation (prenatal) in rats’ offspring caused a significant increase in their serum urea (P<0.003), creatinine, and uric acid compared to the control group (P<0.001) (Tables 3 and 4).
.jpg)

Also, the results of histological studies showed that sodium nitrite consumption during pregnancy and lactation causes a lot of damage to the structure of rats’ offspring renal tissues, such as congestion and diffuse bleeding, damage to the structure of proximal and distal convoluted tubules, dilation, and increase the space around the Bowman capsule, glomerular atrophy, and inflammation around the tubules as well as tubular damage (Figures 1, 2, 3, 4, & 5).
.jpg)
.jpg)
The results of this study showed that sodium nitrite consumption during pregnancy and lactation increases the serum levels of uric acid, urea, and creatinine by damaging the renal tissue structure. These results are consistent with the findings of Rammesh et al. as well as Ramezani Norouzani et al. [18, 19]. Stokes et al. reported that sodium nitrite consumption as a soluble in drinking water increased the amount of nitrite and nitrate in plasma, heart, liver, and kidney [20]. Sodium nitrite causes oxidative stress in the body and produces peroxynitrite (ONOO-), which passes freely through two phospholipid layers of the membrane and reacts with many target molecules such as lipids, proteins, and DNA, and this ultimately leads to cell death through the processes of necrosis and apoptosis [21]. Therefore, in the present study, nitrite compounds may have been transferred to fetuses and neonates of rats through placenta and milk, respectively, and through the above processes caused damage to the renal tissue structure and ultimately increased serum levels of uric acid, urea, and creatinine. It has also been shown that the kidney, as the main site of filtration and one of the detoxification sites in the body, is directly affected by various drugs, and the metabolites produced by the toxins and studies have shown that damage to the renal parenchyma increases the serum concentrations of nitrogen, Blood Urea Nitrogen (BUN), creatinine, and uric acid [22]. Consistent with the histopathological results, Ashrafy et al. reported that a nitrate-containing diet causes microscopic lesions in the kidney’s tissue structure, including hyperemia, cell swelling, and necrosis with moderate to severe renal cell resorption [24]. Consistent with this study’s results, another study showed that the consumption of sodium nitrite in drinking water in male and female rats causes hepatocyte degeneration and necrosis and hemosiderin deposition in the liver, kidney, spleen, and lymph nodes and hemolysis [25]. Besides, Mohseni Kouchesfahani et al. showed that whenever renal function decreases, creatinine, urea, and uric acid levels in the blood increase [26].
On the other hand, the present study results showed that sodium nitrite treatment causes damage to the renal tissue structure, especially in renal glomeruli of animals. Therefore, the increase in serum urea, uric acid, and creatinine in these animals is probably due to the destructive effects of sodium nitrite on the kidneys’ tissue structure. Recent research has clearly shown that nitric oxide can be produced directly from nitrite and impair blood flow to muscles and a greater extent, to other tissues, including the kidneys [27]. One study showed that excess nitrate in drinking water causes disorders in developing various organs of the body, including the fetal liver during pregnancy [28]. The present study had limitations such as high mortality of pregnant rats and their offspring treated with sodium nitrite and their offspring.
On the other hand, the present study results showed that sodium nitrite treatment causes damage to the renal tissue structure, especially in renal glomeruli of animals. Therefore, the increase in serum urea, uric acid, and creatinine in these animals is probably due to the destructive effects of sodium nitrite on the kidneys’ tissue structure. Recent research has clearly shown that nitric oxide can be produced directly from nitrite and impair blood flow to muscles and a greater extent, to other tissues, including the kidneys [27]. One study showed that excess nitrate in drinking water causes disorders in developing various organs of the body, including the fetal liver during pregnancy [28]. The present study had limitations such as high mortality of pregnant rats and their offspring treated with sodium nitrite and their offspring.
5. Conclusion
The results of this study showed that sodium nitrite consumption during pregnancy and lactation causes bleeding and destruction of kidney tissues, cell death, and loss of renal nephrons and possibly increases the serum level of renal factors (creatinine, uric acid, and urea) in the offspring of rats.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Shiraz Branch, Islamic Azad University, Shiraz, (Code: IR.MIAU1395.1016). In this study, all the rights of laboratory animals for human use were observed according to the international protocols of care and use of laboratory animals and was approved by the University Ethics Committee under the number.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Both authors contributed equally in all study procedures.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors of the article must appreciate and thank the esteemed colleagues of Vice Chancellors for Research of the Islamic Azad University, Shiraz Branch, who provided the necessary facilities for this research
References
- Mielnik MB, Aaby K, Skrede G. Commercial antioxidants control lipid oxidation in mechanically deboned turkey meat. Meat Science. 2003; 65(3):1147-55. [DOI:10.1016/S0309-1740(02)00345-5]
- Sebrank JG, Sewalt VJH, Robbins KL, Houser TA. Comparison of a natural Rosemary extract and BHA/BHT for relative antioxidant effectiveness in pork sausage. Meat Science. 2005; 69(2):289-96. [DOI:10.1016/j.meatsci.2004.07.010] [PMID]
- Mary H, Ward Theo M, dek K, Levallois P, Brender J, Gulis G, et al. Workgroup Report: Drinking-water nitrate and health-recent findings and research needs. Environmental Health Perspectives. 2005; 113(11): 1607-1614. [DOI:10.1289/ehp.8043] [PMID] [PMCID]
- Abdel-Hamied AA, Nassar AG, El-Badry N. Investigations on antioxidant and antibacterial activities of some natural extracts. World Journal of Dairy & Food Sciences. 2009; 4(1):1-7. https://www.cabdirect.org/cabdirect/abstract/20103317582
- Georgantelis D, Ambrosiadis I, Katikou P, Blekas G, Georgakis SA. Effect of rosemary extract, chitosan and α-tocopherol on microbiological parameters and lipid oxidation of fresh pork sausages stored at 4°C.Meat Science. 2007; 76(1):172-81 [DOI:10.1016/j.meatsci.2006.10.026] [PMID]
- Yin MC, Cheng WS. Antioxidant and antimicrobial effects of four garlic-derived organosulfur compounds in ground beef. Meat Science. 2003; 63(1):23-8. [DOI:10.1016/S0309-1740(02)00047-5]
- Juibar F, Tavakoli kazerooni A, Ghorbani Ranjbary A. [Histopathological effects of sodium nitrite on the spleen of male and female rats (Persian)]. Iranian South Medical Journal. 2015; 17(6):1160-7. http://ismj.bpums.ac.ir/article-1-632-en.html
- Honikel K-O. The use and control of nitrate and nitrite for the processing of meat products. Meat Science. 2007; 78(1-2):68-76. [DOI:10.1016/j.meatsci.2007.05.030] [PMID]
- Cockburn A, Brambilla G, Fernández ML, Arcella D, Bordajandi LR, Cottrill B, et al. Nitrite in feed: From animal health to human health. Toxicology and Applied pharmacology. 2013; 270(3):209-17 [DOI:10.1016/j.taap.2010.11.008] [PMID]
- Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nature Reviews Drug Discovery. 2008; 7(2):156-67. [DOI:10.1038/nrd2466] [PMID]
- Ismail AETM, Moustafa AM, Abd El-Rhman B G. Microscopic studies of the effect of some food additives on the kidney of albino rat. The Egyptian Journal of Hospital Medicine. 2003; 12(1):12-27. [DOI: 10.12816/EJHM.2003.18241]
- Dezfulian C, Raat N, Shiva S, Gladwin MT. Role of the anion nitrite in ischemia-reperfusion cytoprotection and therapeutics. Cardiovascular Research. 2007; 75(2):327-38. [DOI:10.1016/j.cardiores.2007.05.001] [PMID] [PMCID]
- Stokes KY, Dugas TR, Tang Y, Garg H, Guidry E, Bryan NS. Dietary nitrite prevents hypercholesterolemic microvascular inflammation and reverses endothelial dysfunction. American Journal of Physiology Heart and Circulatory Physiology. 2009; 296(5):H1281-8. [DOI:10.1152/ajpheart.01291.2008] [PMID]
- Juibar F, Khatamsaz S, Ghorbani ranjbry A. [Investigating sodium nitrite effect on blood nitric oxide and histopathologic changes on pulmonary artery in adult male rats (Persian)]. Journal of Shahid Sadoughi University of Medical Sciences and Health Services. 2013; 21(5):609-18. https://www.sid.ir/en/Journal/ViewPaper.aspx?ID=356450
- Roussel AM, Hininger I, Benaraba R, Ziegenfuss TN, Anderson RA. Antioxidant effects of a cinnamon extract in people with impaired fasting glucose that are overweight or obese. Journal of the American College of Nutrition. 2009, 28(1):16-21. [DOI:10.1080/07315724.2009.10719756] [PMID]
- Li J, Li W, Su J, Liu W, Altura BT, Altura BM. Peroxynitrite induces apoptosis in rat aortic smooth muscle cells: possible relation to vascular diseases. Experimental Biology and Medicine (Maywood, N.J.). 2004; 229(3):264-9. [DOI:10.1177/153537020422900307] [PMID]
- Matthew JA , Evie C, Mark TG, Edith T, Brian SZ. Dietary nitrates and nitrites modulate vascular intimal hyperplasia. Journal of the American College of Surgeons. 2010; 211(3):S138. DOI: [DOI:10.1016/j.jamcollsurg.2010.06.368]
- Ramezani Noroozani F, Ojinejad D, Ghorbani Ranjbary A. [Effects of sodium nitrite on Liver enzymes and histological structure of liver in streptozotocin-induced diabetic rats (Persian)]. Journal of Mazandaran University of Medical Sciences. 2017; 26(144):171-9. https://www.sid.ir/en/journal/ViewPaper.aspx?id=536705
- Remmesh B, Viswanathan P, Pugalendi KV. Protective effect of Vmbeliferone on membranous fatty acid composition in streptozotocin-induced diabetic rats. European Journal of Pharmacology. 2007; 566(1-3):231-9. [DOI:10.1016/j.ejphar.2007.03.045] [PMID]
- Stokes KY, Dugas TR, Tang Y, Garg H, Guidy E, Bryan NS. Dietary nitrite prevents hypercholesterolemic microvascular inflammation and reverses endothelial dysfunction. American Journal of Physiology. Heart and Circulatory Physiology. 2009; 296(5):1281-8. [DOI:10.1152/ajpheart.01291.2008] [PMID]
- Gad SB, Zaghloul DM. Beneficial effects of green tea extract on liver and kidney function, ultrastructure, lipid profile and hematological parameters in aged male rats. Global Veterinaria. 2013; 11(2):191-205. [DOI: 10.5829/idosi.gv.2013.11.2.7472]
- Ashrafy E, Hosseini SE. [Protective effects of Cinnamon hydro-alcoholic extract on liver lesions induced non-alcoholic fatty liver disease and sodium nitrite poisoning in adult male rats (Persian)]. Journal of Shahrekord University of Medical Sciences. 2018; 19(6):13-23. http://eprints.skums.ac.ir/6908/
- Roussel A-M, Hiniger I, Benaraba R, Ziegen fuss TN, Anderson RA. Antioxidant effects of a Cinnamon extract in people with impaired fasting glucose that are over weight or obese . Journal of the American College of Nutrition. 2009; 28(1):16-21. [DOI:10.1080/07315724.2009.10719756] [PMID]
- Mohseni Kouchesfhani H, Khoshnoud S, Nabiuni M. [Evaluation of protective effect of hydro-alcoholic extract of raspberry fruit on the methotrexate-induced nephrotoxicity in male Wistar rats (Persian)]. Razi Journal of Medical Sciences. 2015; 22(138):78-87. http://rjms.iums.ac.ir/article-1-4089-en.html
- Zand J, Lanza F, Garg HK, Bryan NS. All-natural nitrite and nitrate containing dietary supplement promotes nitric oxide production and reduces triglycerides in humans. Nutrition Research (New York, N.Y.). 2011; 31(4):262-9. [DOI:10.1016/j.nutres.2011.03.008] [PMID]
- Ghoreishi M, Nabuni M, ShiraviA A, Rostami M, Karimzadeh Barde L. [The effects of the water nitrate on the histology and immunohistology of the liver development in NMRI Mice Fetus (Persian)]. Journal Of Animal Research (Iranian Journal of Biology).2016;29(2):215-222. https://animal.ijbio.ir/article_832.html?lang=en
Type of Study: Original |
Subject:
Basic Medical Science
Received: 2020/03/29 | Accepted: 2020/10/6 | Published: 2021/04/1
Received: 2020/03/29 | Accepted: 2020/10/6 | Published: 2021/04/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






.jpg)