Volume 28, Issue 4 (Autumn 2022)
Intern Med Today 2022, 28(4): 530-541 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Salehi M, Nasimi A, Ghasemi H, Nezami H, Hassanzadeh Haghighi F, Fani M. Seroprevalence of TORCH Syndrome Among Women of Reproductive Age in Mashhad, Northeast of Iran. Intern Med Today 2022; 28 (4) :530-541
URL: http://imtj.gmu.ac.ir/article-1-3924-en.html
URL: http://imtj.gmu.ac.ir/article-1-3924-en.html
Mitra Salehi1 

 , Ali Nasimi *
, Ali Nasimi * 

 2, Hamed Ghasemi3
2, Hamed Ghasemi3 

 , Hossein Nezami4
, Hossein Nezami4 
 , Faria Hassanzadeh Haghighi5
, Faria Hassanzadeh Haghighi5 

 , Mona Fani6
, Mona Fani6 




 , Ali Nasimi *
, Ali Nasimi * 

 2, Hamed Ghasemi3
2, Hamed Ghasemi3 

 , Hossein Nezami4
, Hossein Nezami4 
 , Faria Hassanzadeh Haghighi5
, Faria Hassanzadeh Haghighi5 

 , Mona Fani6
, Mona Fani6 


1- Vector-borne Diseases Research Center, North Khorasan University of Medical Sciences, Bojnurd, Iran
2- Infectious Diseases Research Center, student research committee, Faculty of Medicine, Gonabad University of Medical Sciences, Gonabad, Iran. , alsnasimi@gmail.com
3- Infectious Diseases Research Center, student research committee, Faculty of Medicine, Gonabad University of Medical Sciences, Gonabad, Iran.
4- Department of Epidemiology and Biostatistics, Student Research Committee, School of Health, Mashhad University of Medical Sciences, Mashhad, Iran.
5- Department of Bacteriology and Virology, Mashhad University of Medical Sciences, Mashhad, Iran.
6- Vector-borne Diseases Research Center, North Khorasan University of Medical Sciences, Bojnurd, Iran.
2- Infectious Diseases Research Center, student research committee, Faculty of Medicine, Gonabad University of Medical Sciences, Gonabad, Iran. , alsnasimi@gmail.com
3- Infectious Diseases Research Center, student research committee, Faculty of Medicine, Gonabad University of Medical Sciences, Gonabad, Iran.
4- Department of Epidemiology and Biostatistics, Student Research Committee, School of Health, Mashhad University of Medical Sciences, Mashhad, Iran.
5- Department of Bacteriology and Virology, Mashhad University of Medical Sciences, Mashhad, Iran.
6- Vector-borne Diseases Research Center, North Khorasan University of Medical Sciences, Bojnurd, Iran.
Full-Text [PDF 3313 kb]
(294 Downloads)
| Abstract (HTML) (518 Views)
Full-Text: (182 Views)
Introduction
Congenital defects are a worldwide concern. It is also the primary cause of mortality in infants in both developing and developed countries. A variety of abnormalities are due to genetic inheritance and environmental factors. Infection during pregnancy plays a significant role in the development of major birth defects. One of these infections is toxoplasmosis, other agents, rubella, cytomegalovirus, and herpes simplex (TORCH) syndrome which will not harm the fetus if detected and treated promptly [1, 2, 3]. The term TORCH refers to infectious agents that can be transmitted to a child by vertical infection, either intrauterine or post-natal. Primary infections associated with some TORCH pathogens during pregnancy, especially in the first trimester, are associated with an increased risk of miscarriage, stillbirth, sterilization, preterm delivery, congenital anomalies, and transient or chronic fatal disease. The risk of this disorder is dependent on gestational age and pathogens [4]. Primary infections are more harmful compared to secondary infections during pregnancy [5]. TORCH syndrome can lead to spontaneous abortion or severe birth defects in the fetus [2, 3]. This infection is responsible for 2% to 3% of all congenital anomalies. Mental retardation is one of the major complications that will impose a significant economic burden on society and the family [6, 7].
TORCH is associated with several factors and occurs at various stages of pregnancy. Toxoplasma gondii (T. gondii), rubella virus (RV), cytomegalovirus (CMV), and herpes simplex virus (HSV) types 1 and 2 are known as the TORCH syndrome that leads to neonatal mortality worldwide [8, 9, 10]. The majority of these pathogens do not affect the mother؛ however, they have disastrous consequences for the fetus [10, 11]. Accordingly, it will be difficult to diagnose this syndrome in pregnant mothers. Measuring immunoglobulin M (IgM) antibodies against TORCH is a way to detect an early infection [12].
If a growing fetus becomes infected with a TORCH agent it may cause a miscarriage, stillbirth, fetal growth retardation (intrauterine growth retardation), or congenital malformation. Lethargy, fever, nutritional problems, hepatosplenomegaly, and low hemoglobin levels are some of the symptoms and clinical findings in newborns infected with TORCH agents. Moreover, petechiae or purpura may develop on the skin in infected infants. Yellow skin, whites of the eyes hemolytic anemia, thrombocytopenia, jaundice, and chorioretinitis can be observed in infected infants. Other abnormalities may occur, depending on the fetal development stage at the time of infection and the severity of the infection [13, 14].
According to different results obtained from screening experiments, the prevalence of TORCH factors is dependent on geographical area, economics, and culture [15, 16].
T. gondii is a parasitic infection and zoonotic disease that cause TORCH syndrome. TORCH syndrome symptoms range from asymptomatic findings to chorioretinitis [17], hearing impairment, hydrocephalus, and fetal psychomotor disorders [18]. Toxoplasma infection is prevalent in China due to its special diet [19]. Because of the adverse effects of this infection on the fetus, it is critical to diagnose infected women before pregnancy to prevent fetal disorders.
RV is a viral disease with mild or asymptomatic and even catastrophic consequences in the fetus [14]. RV can transmit through respiration and mother-fetal vertical transmission [20]. This is one of the most important causes of TORCH syndrome. In the first 12 weeks, the fetal infection rate exceeds 80%, to be reduced to 25% by the end of the second quarter. On 27 to 30 weeks, it may increase up to 35% [14].
CMV is one of the major causes of congenital anomalies in newborns and is associated with severe fetal abnormalities, such as chorioretinitis, sensorineural deafness, and cerebral palsy [21, 22].
The prevalence of this syndrome varies based on geographical area؛ however, Southeast Asia and sub-Saharan Africa have the highest mortality rates from these infections [23]. A general national study that involves TORCH effects on pregnant women and investigates whether they are associated with adverse pregnancy outcomes is not available, to the best of our knowledge. Since our country is also in a high-risk region, research in this field is necessary. The present study aims to determine the prevalence of TORCH infection in Mashhad City, Iran in women of their reproductive age.
Materials and Methods
Study population
This study evaluated the serological records of 417 women of reproductive age who were admitted to 3 laboratories in Mashhad City, Iran from April 2016 to March 2020. The subjects were women of reproductive age with a mean age of 30. 8±5. 77 years, with a minimum age of 17 years and a maximum age of 47 years.
Determination of cytomegalovirus infection
Immunoglobulin G (IgG) and IgM antibodies against T. gondii, CMV, and RV were assayed by the enzyme-linked immunosorbent assay (ELISA) kit (Pishtazteb, Tehran, Iran).
Data analysis
Statistical data were analyzed by the SPSS software, version 23 along with the chi-square test. A 95% confidence interval (CI) was estimated for each TORCH agent in the positive patients.
Results
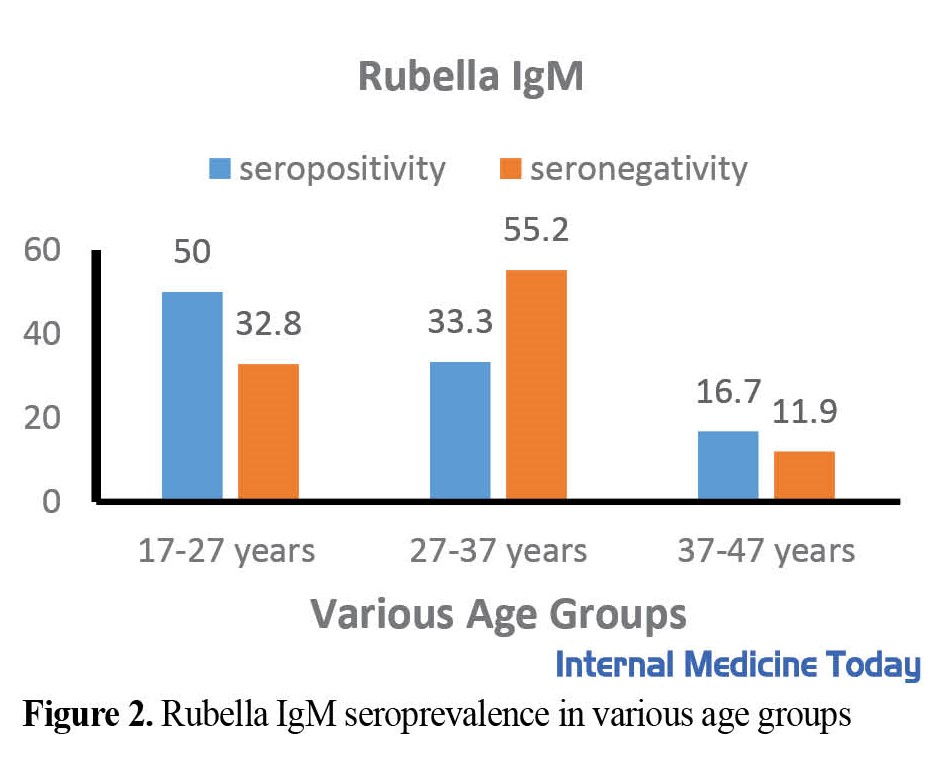
To determine the seroprevalence of TORCH infection, 417 women of reproductive age between 17 to 47 years with a mean age of 30. 8±5. 77 were included. The study found that a significant proportion of the participants had antibodies against T. gondii, Rubella, and CMV. Specifically, 19.2% of the participants had anti-T. gondii IgG antibodies, while 94.5% and 96.4% had anti-Rubella IgG and anti-CMV IgG, respectively. A small percentage of participants had IgM antibodies against these infections. The prevalence of TORCH IgG and IgM was determined in 417 women of reproductive age as shown in Table 1 and Figure 1, 2, 3, 4, 5, 6, which also illustrates the prevalence of TORCH infection in three different age groups.
.jpg)
The relationship between age and anti-T. gondii IgG was significant (P<0.05), as it was 6. 44 times higher in the age group of 37-47 years compared with the age group of 17-27 years (Table 2)؛ this is probable due to more exposure to the pathogen. These results were not significant for RV.

Discussion
There are comparatively less data on the exact spread of TORCH infections among reproductive-age women in different regions. TORCH syndrome can be asymptomatic in women؛ therefore, it is critical to diagnose the infected women to prevent fetal complications [21, 22]. This study aimed to investigate the prevalence of TORCH syndrome in women of reproductive age in Mashhad City, Iran.
The seroprevalence of T. gondii is prevalent in tropical countries and in areas where raw or semi-raw meat or contaminated food or water with cat fecal oocysts is consumed [24, 25, 26]. For example, in India, the prevalence of anti-T. gondii IgG in women of reproductive age has been reported in the range of 25% to 28% [27, 28].
In previous studies in Iran, the seroprevalence of T. gondii infection was reported at 35% to 41% in the general population [29]. Additionally, a study conducted on 2726 women of reproductive age in Tabriz reported that 26.5% of them had T. gondii IgG antibodies, while only 0.4% had IgM antibodies [30]. However, in our study, 19. 2% of cases were positive for IgG T. gondii, and 1. 9 % for IgM T. gondii. It may be due to the different diet habits, geography, and socio-economic characteristics of populations.
CMV is one of the most common viruses that can cause congenital infections worldwide [31]. According to prior studies in Iran, Mashhad City had one of the highest prevalence rates of CMV IgG (99%) and IgM (0.25%) [32]. The current study indicated that the anti-CMV IgG and IgM antibodies were 96.4% and 1.6%, respectively. As a result, it is critical to diagnose CMV in women of childbearing age to prevent fetal defects [33].
A maternal screening of TORCH infection study was conducted on cases of fetal growth restriction in Japan, which concluded that only the CMV antibodies test can be considered in maternal serum [34].
Although, RV infection is a harmless dermatitis disease in childhood, during pregnancy (especially in the first 12 weeks) can result in congenital rubella syndrome, severe birth defects, and miscarriage [35]. Depending on the success of vaccination programs, the seroprevalence of RV antibodies among reproductive-age women is different in each country. For example, in India, 83. 4% were seropositive for rubella IgG antibodies, and 94% of Turkish women were seropositive [36, 37].
A study conducted in Tabriz City, Iran revealed that 89. 4% of women had RV IgG antibodies and 0. 5% had IgM antibodies [30]. In the present study, the rubella IgG rate was 94.5%, which shows that we were more successful than other areas of Iran in vaccination programs؛ however, we still have to spread our coverage.
According to our study, the seroprevalence of TORCH syndrome among women of reproductive age in Mashhad City, Iran was relatively low؛ however, it varies in different geographic areas.
Although TORCH syndrome can cause a mild illness in women, intrauterine infections during pregnancy can lead to serious complications in the fetus. Hence, during pregnancy and at childbearing age, serological tests (titers of both IgM and IgG antibodies) are essential to screen and prevent congenital malformations [38].
Conclusion
Although seroprevalence of TORCH syndrome among women of reproductive age in Mashhad City, Iran was relatively low, to avoid unfavorable fetal outcomes, all women of reproductive age should be screened for the TORCH complex.
Study Limitations
Our study was conducted on a limited number of samples and only in the City of Mashhad, Iran. Also, there were little laboratory data on HSV infection as one of the pathogens of TORCH syndrome. Expanding this study throughout the country and understanding the main causes of this syndrome in Iran will help to alleviate concerns about the percentage of congenital defects with infectious causes.
Ethical Considerations
Compliance with ethical guidelines
Ethical approval was obtained from the Ethics Committee of Gonabad University of Medical Sciences (Code: IR.GMU.REC.1398.009). Informed consent was obtained from all participants. Their personal information was kept confidential.
Funding
This study was funded by the Student Research Committee of Gonabad University of Medical Sciences.
Authors' contributions
Conceptualization and project administration: Ali Nasimi and Mitra Salehi; Data collection and analysis: Hamed Ghasemi, Hossein Nezami, and Faria Hassanzadeh Haghighi; Writing the original version, editing & review: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Reference:
Congenital defects are a worldwide concern. It is also the primary cause of mortality in infants in both developing and developed countries. A variety of abnormalities are due to genetic inheritance and environmental factors. Infection during pregnancy plays a significant role in the development of major birth defects. One of these infections is toxoplasmosis, other agents, rubella, cytomegalovirus, and herpes simplex (TORCH) syndrome which will not harm the fetus if detected and treated promptly [1, 2, 3]. The term TORCH refers to infectious agents that can be transmitted to a child by vertical infection, either intrauterine or post-natal. Primary infections associated with some TORCH pathogens during pregnancy, especially in the first trimester, are associated with an increased risk of miscarriage, stillbirth, sterilization, preterm delivery, congenital anomalies, and transient or chronic fatal disease. The risk of this disorder is dependent on gestational age and pathogens [4]. Primary infections are more harmful compared to secondary infections during pregnancy [5]. TORCH syndrome can lead to spontaneous abortion or severe birth defects in the fetus [2, 3]. This infection is responsible for 2% to 3% of all congenital anomalies. Mental retardation is one of the major complications that will impose a significant economic burden on society and the family [6, 7].
TORCH is associated with several factors and occurs at various stages of pregnancy. Toxoplasma gondii (T. gondii), rubella virus (RV), cytomegalovirus (CMV), and herpes simplex virus (HSV) types 1 and 2 are known as the TORCH syndrome that leads to neonatal mortality worldwide [8, 9, 10]. The majority of these pathogens do not affect the mother؛ however, they have disastrous consequences for the fetus [10, 11]. Accordingly, it will be difficult to diagnose this syndrome in pregnant mothers. Measuring immunoglobulin M (IgM) antibodies against TORCH is a way to detect an early infection [12].
If a growing fetus becomes infected with a TORCH agent it may cause a miscarriage, stillbirth, fetal growth retardation (intrauterine growth retardation), or congenital malformation. Lethargy, fever, nutritional problems, hepatosplenomegaly, and low hemoglobin levels are some of the symptoms and clinical findings in newborns infected with TORCH agents. Moreover, petechiae or purpura may develop on the skin in infected infants. Yellow skin, whites of the eyes hemolytic anemia, thrombocytopenia, jaundice, and chorioretinitis can be observed in infected infants. Other abnormalities may occur, depending on the fetal development stage at the time of infection and the severity of the infection [13, 14].
According to different results obtained from screening experiments, the prevalence of TORCH factors is dependent on geographical area, economics, and culture [15, 16].
T. gondii is a parasitic infection and zoonotic disease that cause TORCH syndrome. TORCH syndrome symptoms range from asymptomatic findings to chorioretinitis [17], hearing impairment, hydrocephalus, and fetal psychomotor disorders [18]. Toxoplasma infection is prevalent in China due to its special diet [19]. Because of the adverse effects of this infection on the fetus, it is critical to diagnose infected women before pregnancy to prevent fetal disorders.
RV is a viral disease with mild or asymptomatic and even catastrophic consequences in the fetus [14]. RV can transmit through respiration and mother-fetal vertical transmission [20]. This is one of the most important causes of TORCH syndrome. In the first 12 weeks, the fetal infection rate exceeds 80%, to be reduced to 25% by the end of the second quarter. On 27 to 30 weeks, it may increase up to 35% [14].
CMV is one of the major causes of congenital anomalies in newborns and is associated with severe fetal abnormalities, such as chorioretinitis, sensorineural deafness, and cerebral palsy [21, 22].
The prevalence of this syndrome varies based on geographical area؛ however, Southeast Asia and sub-Saharan Africa have the highest mortality rates from these infections [23]. A general national study that involves TORCH effects on pregnant women and investigates whether they are associated with adverse pregnancy outcomes is not available, to the best of our knowledge. Since our country is also in a high-risk region, research in this field is necessary. The present study aims to determine the prevalence of TORCH infection in Mashhad City, Iran in women of their reproductive age.
Materials and Methods
Study population
This study evaluated the serological records of 417 women of reproductive age who were admitted to 3 laboratories in Mashhad City, Iran from April 2016 to March 2020. The subjects were women of reproductive age with a mean age of 30. 8±5. 77 years, with a minimum age of 17 years and a maximum age of 47 years.
Determination of cytomegalovirus infection
Immunoglobulin G (IgG) and IgM antibodies against T. gondii, CMV, and RV were assayed by the enzyme-linked immunosorbent assay (ELISA) kit (Pishtazteb, Tehran, Iran).
Data analysis
Statistical data were analyzed by the SPSS software, version 23 along with the chi-square test. A 95% confidence interval (CI) was estimated for each TORCH agent in the positive patients.
Results
To determine the seroprevalence of TORCH infection, 417 women of reproductive age between 17 to 47 years with a mean age of 30. 8±5. 77 were included. The study found that a significant proportion of the participants had antibodies against T. gondii, Rubella, and CMV. Specifically, 19.2% of the participants had anti-T. gondii IgG antibodies, while 94.5% and 96.4% had anti-Rubella IgG and anti-CMV IgG, respectively. A small percentage of participants had IgM antibodies against these infections. The prevalence of TORCH IgG and IgM was determined in 417 women of reproductive age as shown in Table 1 and Figure 1, 2, 3, 4, 5, 6, which also illustrates the prevalence of TORCH infection in three different age groups.
.jpg)
The relationship between age and anti-T. gondii IgG was significant (P<0.05), as it was 6. 44 times higher in the age group of 37-47 years compared with the age group of 17-27 years (Table 2)؛ this is probable due to more exposure to the pathogen. These results were not significant for RV.

Discussion
There are comparatively less data on the exact spread of TORCH infections among reproductive-age women in different regions. TORCH syndrome can be asymptomatic in women؛ therefore, it is critical to diagnose the infected women to prevent fetal complications [21, 22]. This study aimed to investigate the prevalence of TORCH syndrome in women of reproductive age in Mashhad City, Iran.
The seroprevalence of T. gondii is prevalent in tropical countries and in areas where raw or semi-raw meat or contaminated food or water with cat fecal oocysts is consumed [24, 25, 26]. For example, in India, the prevalence of anti-T. gondii IgG in women of reproductive age has been reported in the range of 25% to 28% [27, 28].
In previous studies in Iran, the seroprevalence of T. gondii infection was reported at 35% to 41% in the general population [29]. Additionally, a study conducted on 2726 women of reproductive age in Tabriz reported that 26.5% of them had T. gondii IgG antibodies, while only 0.4% had IgM antibodies [30]. However, in our study, 19. 2% of cases were positive for IgG T. gondii, and 1. 9 % for IgM T. gondii. It may be due to the different diet habits, geography, and socio-economic characteristics of populations.
CMV is one of the most common viruses that can cause congenital infections worldwide [31]. According to prior studies in Iran, Mashhad City had one of the highest prevalence rates of CMV IgG (99%) and IgM (0.25%) [32]. The current study indicated that the anti-CMV IgG and IgM antibodies were 96.4% and 1.6%, respectively. As a result, it is critical to diagnose CMV in women of childbearing age to prevent fetal defects [33].
A maternal screening of TORCH infection study was conducted on cases of fetal growth restriction in Japan, which concluded that only the CMV antibodies test can be considered in maternal serum [34].
Although, RV infection is a harmless dermatitis disease in childhood, during pregnancy (especially in the first 12 weeks) can result in congenital rubella syndrome, severe birth defects, and miscarriage [35]. Depending on the success of vaccination programs, the seroprevalence of RV antibodies among reproductive-age women is different in each country. For example, in India, 83. 4% were seropositive for rubella IgG antibodies, and 94% of Turkish women were seropositive [36, 37].
A study conducted in Tabriz City, Iran revealed that 89. 4% of women had RV IgG antibodies and 0. 5% had IgM antibodies [30]. In the present study, the rubella IgG rate was 94.5%, which shows that we were more successful than other areas of Iran in vaccination programs؛ however, we still have to spread our coverage.
According to our study, the seroprevalence of TORCH syndrome among women of reproductive age in Mashhad City, Iran was relatively low؛ however, it varies in different geographic areas.
Although TORCH syndrome can cause a mild illness in women, intrauterine infections during pregnancy can lead to serious complications in the fetus. Hence, during pregnancy and at childbearing age, serological tests (titers of both IgM and IgG antibodies) are essential to screen and prevent congenital malformations [38].
Conclusion
Although seroprevalence of TORCH syndrome among women of reproductive age in Mashhad City, Iran was relatively low, to avoid unfavorable fetal outcomes, all women of reproductive age should be screened for the TORCH complex.
Study Limitations
Our study was conducted on a limited number of samples and only in the City of Mashhad, Iran. Also, there were little laboratory data on HSV infection as one of the pathogens of TORCH syndrome. Expanding this study throughout the country and understanding the main causes of this syndrome in Iran will help to alleviate concerns about the percentage of congenital defects with infectious causes.
Ethical Considerations
Compliance with ethical guidelines
Ethical approval was obtained from the Ethics Committee of Gonabad University of Medical Sciences (Code: IR.GMU.REC.1398.009). Informed consent was obtained from all participants. Their personal information was kept confidential.
Funding
This study was funded by the Student Research Committee of Gonabad University of Medical Sciences.
Authors' contributions
Conceptualization and project administration: Ali Nasimi and Mitra Salehi; Data collection and analysis: Hamed Ghasemi, Hossein Nezami, and Faria Hassanzadeh Haghighi; Writing the original version, editing & review: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Reference:
- Gomathi S, Latha TP. Case report on torch syndrome with multiple anomalies in neonate. International Journal of Nursing Education and Research. 2018؛ 6(1): 12-4. [DOI: 10. 5958/2454-2660. 2018. 00003. 0]
- Adgoy ET, Elfatih M, Elhadi B, Zerizgie H, Said SM, Tekle F, et al. Seroprevalence of TORCH in women with spontaneous abortion and stillbirth, in Asmara, Eritrea. Population Medicine. 2020؛ 20(1): 36. [DOI: 10. 18332/popmed/128008]
- Naame ZK, Thuwaini MM, Mahdi DS. Seroprevalence of (Toxoplasma gondii, CMV, Rubella and HSV-1&2) in aborted women in Basra, southern of Iraq. Annals of Tropical Medicine and Public Health. 2021; 24(05). [Link]
- de Jong EP, Vossen AC, Walther FJ, Lopriore E. How to use... neonatal TORCH testing. Archives of Disease in Childhood-Education and Practice. 2013؛ 98(3): 93-8. [DOI: 10. 1136/archdischild-2012-303327] [PMID]
- Rasti S, Ghasemi FS, Abdoli A, Piroozmand A, Mousavi SG, Fakhrie-Kashan Z. ToRCH “co-infections” are associated with increased risk of abortion in pregnant women. Congenital Anomalies. 2016؛ 56(2): 73-8. [DOI: 10. 1111/cga. 12138] [PMID]
- Neu N, Duchon J, Zachariah P. TORCH infections. Clinics in Perinatology. 2015؛ 42(1): 77-103. [DOI: 10. 1016/j. clp. 2014. 11. 001] [PMID]
- Arora N, Sadovsky Y, Dermody TS, Coyne CB. Microbial vertical transmission during human pregnancy. Cell Host & Microbe. 2017؛ 21(5): 561-7. [DOI: 10. 1016/j. chom. 2017. 04. 007] [PMID]
- Sunitha T, Prasoona KR, Kumari TM, Srinadh B, Deepika ML, Aruna R, et al. Risk factors for congenital anomalies in high-risk pregnant women: A large study from South India. Egyptian Journal of Medical Human Genetics. 2017؛ 18(1): 79-85. [DOI: 10. 1016/j. ejmhg. 2016. 04. 001]
- Epps RE, Pittelkow MR, Su WP. TORCH syndrome. Seminars in Dermatology. 1995؛ 14(2): 179-86. [DOI: 10. 1016/S1085-5629(05)80016-1] [PMID]
- Sahu SK, Pradhan SK, Nayak LM. Seroprevalence of TORCH infection among pregnant women. International Journal Of Community Medicine And Public Health. 2019؛ 6(5): 2189–94. [DOI: 10. 18203/2394-6040. ijcmph20191842]
- Sadik MS, Fatima H, Jamil K, Patil C. Study of TORCH profile in patients with bad obstetric history. Biology and Medicine. 2012؛ 4(2): 95-101. [Link]
- Coyne CB, Lazear HM. Zika virus-reigniting the TORCH. Nature Reviews Microbiology. 2016؛ 14(11): 707-15. [DOI: 10. 1038/nrmicro. 2016. 125] [PMID]
- Kostrzewski MS. National organization of rare disorders (NORD) web site. Journal of Consumer Health on the Internet. 2006؛ 10(1): 77-87. [DOI: 10. 1300/J381v10n01_06]
- Leung KKY, Hon KL, Yeung A, Leung AKC, Man E. Congenital infections in Hong Kong: An overview of TORCH. Hong Kong Medical Journal. 2020؛ 26(2): 127-38. [DOI: 10. 12809/hkmj198287] [PMID]
- Duran B, et al. Doğum öncesi bakımda tartışmalı bir konu: TORCH taraması. CÜ Tıp Fakültesi Dergisi. 2002; 24(4): 185-90.
- Karabulut A, Polat Y, Türk M, Balci YI. Evaluation of rubella, Toxoplasma gondii, and cytomegalovirus seroprevalences among pregnant women in Denizli province. Turkish Journal of Medical Sciences. 2011؛ 41(1): 159-64. [DOI: 10. 3906/sag-1001-568]
- Grewal DS. Infectious retinitis: TORCH Syndrome. In: Toth CA, Ong SS, editors. Handbook of pediatric retinal OCT and the eye-brain connection. Amsterdam: Elsevier؛ 2020. [DOI: 10. 1016/B978-0-323-60984-5. 00034-2]
- Patel KK, Shrivastava G, Bhatambare G, Bajpai T. Antenatal detection of IgM and IgG antibodies to Toxoplasma gondii in a hospital from central India. International Journal of Health System and Disaster Management. 2014؛ 2(3): 133. [DOI: 10. 4103/2347-9019. 142188]
- Qi Y, Zhu S, Li C, Wu H, Yue H, Zhang Y, et al. Seroepidemiology of TORCH antibodies in the reproductive-aged women in China. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2020؛ 254: 114-8. [DOI: 10. 1016/j. ejogrb. 2020. 09. 010] [PMID]
- Leung AKC, Hon KL, Leong KF. Rubella (German measles) revisited. Hong Kong Medical Journal. 2019؛ 25(2): 134-41. [DOI: 10. 12809/hkmj187785] [PMID]
- Turbadkar D, Mathur M, Rele M. Seroprevalence of torch infection in bad obstetric history. Indian Journal of Medical Microbiology. 2003؛ 21(2): 108-10. [DOI: 10. 1016/S0255-0857(21)03131-5] [PMID]
- Sen MR, Shukla BN, Tuhina B. Prevalence of serum antibodies to TORCH infection in and around Varanasi, Northern India. Journal of Clinical and Diagnostic Research: JCDR. 2012؛ 6(9): 1483-5. [DOI: 10. 7860/JCDR/2012/4550. 2538] [PMID]
- Lawn JE, Yakoob MY, Haws RA, Soomro T, Darmstadt GL, Bhutta ZA. 3. 2 million stillbirths: Epidemiology and overview of the evidence review. BMC Pregnancy and Childbirth. 2009؛ 9 Suppl 1(Suppl 1): S2. [DOI: 10. 1186/1471-2393-9-S1-S2] [PMID]
- McAuley JB. Congenital toxoplasmosis. Journal of the Pediatric Infectious Diseases Society. 2014؛ 3(suppl_1): S30-5. [DOI: 10. 1093/jpids/piu077] [PMID]
- Singh S. Congenital toxoplasmosis: Clinical features, outcomes, treatment, and prevention. Tropical Parasitology. 2016؛ 6(2): 113-22. [DOI: 10. 4103/2229-5070. 190813] [PMID]
- Batra P, Batra M, Singh S. Epidemiology of TORCH infections and understanding the serology in their diagnosis. Journal of Fetal Medicine. 2020؛ 7(1): 25-9. [DOI: 10. 1007/s40556-019-00232-8]
- Singh S, Munawwar A, Rao S, Mehta S, Hazarika NK. Serologic prevalence of Toxoplasma gondii in Indian women of child bearing age and effects of social and environmental factors. PLoS Neglected Tropical Diseases. 2014؛ 8(3): e2737. [DOI: 10. 1371/journal. pntd. 0002737] [PMID]
- Colombo FA, Vidal JE, Penalva de Oliveira AC, Hernandez AV, Bonasser-Filho F, Nogueira RS, et al. Diagnosis of cerebral toxoplasmosis in AIDS patients in Brazil: Importance of molecular and immunological methods using peripheral blood samples. Journal of Clinical Microbiology. 2005؛ 43(10): 5044-7. [DOI: 10. 1128/JCM. 43. 10. 5044-5047. 2005] [PMID]
- Daryani A, Sarvi S, Aarabi M, Mizani A, Ahmadpour E, Shokri A, et al. Seroprevalence of Toxoplasma gondii in the Iranian general population: A systematic review and meta-analysis. Acta Tropica. 2014؛ 137: 185-94. [DOI: 10. 1016/j. actatropica. 2014. 05. 015] [PMID]
- Nabizadeh E, Ghotaslou A, Salahi B, Ghotaslou R. The screening of Rubella Virus, Cytomegalovirus, Hepatitis B Virus, and Toxoplasma gondii antibodies in prepregnancy and reproductive-age women in Tabriz, Iran. 2022؛ 2022: 4490728. [DOI: 10. 1155/2022/4490728] [PMID]
- Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The “silent” global burden of congenital cytomegalovirus. Clinical Microbiology Reviews. 2013؛ 26(1): 86-102. [DOI: 10. 1128/CMR. 00062-12] [PMID]
- Sharghi M, Musavi H, Mansurkhani SM, Kooti W, Behzadifar M, Ashrafi-Zadeh H, et al. Seroprevalence of cytomegalovirus among women of reproductive age in Iran: A Systematic review and meta-analysis. Iranian Journal of Public Health. 2019؛ 48(2): 206-16. [PMID]
- Rawlinson WD, Boppana SB, Fowler KB, Kimberlin DW, Lazzarotto T, Alain S, et al. Congenital cytomegalovirus infection in pregnancy and the neonate: Consensus recommendations for prevention, diagnosis, and therapy. The Lancet Infectious Diseases. 2017؛ 17(6): e177-88. [DOI: 10. 1016/S1473-3099(17)30143-3] [PMID]
- Yamamoto R, Ishii K, Shimada M, Hayashi S, Hidaka N, Nakayama M, et al. Significance of maternal screening for toxoplasmosis, rubella, cytomegalovirus and herpes simplex virus infection in cases of fetal growth restriction. Journal of Obstetrics and Gynaecology Research. 2013؛ 39(3): 653-7. [DOI: 10. 1111/j. 1447-0756. 2012. 02012. x] [PMID]
- Ghaderi R, Ghaderi F. Rubella immunity among pregnant women in Iran. MOJ Immunology. 2016؛ 4(2): 00118. [DOI: 10. 15406/moji. 2016. 04. 00118]
- Muliyil DE, Singh P, Jois SK, Otiv S, Suri V, Varma V, et al. Sero-prevalence of rubella among pregnant women in India, 2017. Vaccine. 2018؛ 36(52): 7909-12. [DOI: 10. 1016/j. vaccine. 2018. 11. 013] [PMID]
- Tamer GS, Dundar D, Caliskan E. Seroprevalence of Toxoplasma gondii, rubella and cytomegalovirus among pregnant women in western region of Turkey. Clinical and Investigative Medicine. 2009؛ 32(1): E43-7. [DOI: 10. 25011/cim. v32i1. 5086] [PMID]
- Numan O, Vural F, Aka N, Alpay M, Coskun AD. TORCH seroprevalence among patients attending obstetric Care Clinic of Haydarpasa Training and Research Hospital affiliated to association of Istanbul northern Anatolia public hospitals. Northern Clinics of Istanbul. 2015؛ 2(3): 203-9. [DOI: 10. 14744/nci. 2015. 55376] [PMID]
Type of Study: Original |
Subject:
Obstetrics and Gynecology
Received: 2022/08/22 | Accepted: 2023/09/12 | Published: 2022/09/23
Received: 2022/08/22 | Accepted: 2023/09/12 | Published: 2022/09/23
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |