Volume 28, Issue 4 (Autumn 2022)
Intern Med Today 2022, 28(4): 448-463 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Kazemi A, Kerendi H, Naghizadeh S. Investigating the Effects of Four Weeks of Spinal Nerve Ligation After a Period of Combined Training on the Expression of Involved Genes in Calcium Current in Plantaris Muscle of Male Wistar Rats. Intern Med Today 2022; 28 (4) :448-463
URL: http://imtj.gmu.ac.ir/article-1-3893-en.html
URL: http://imtj.gmu.ac.ir/article-1-3893-en.html
1- Department of Sports Sciences, Faculty of Literature and Humanities, Vali-e-Asr University, Rafsanjan, Iran. , a.kazemi@vru.ac.ir
2- Department of Physical Education and Sports Sciences, Faculty of Literature and Humanities, Lorestan University, Khorramabad, Iran.
3- Department of Sports Sciences, Faculty of Literature and Humanities, Kerman Branch, Islamic Azad University, Kerman, Iran.
2- Department of Physical Education and Sports Sciences, Faculty of Literature and Humanities, Lorestan University, Khorramabad, Iran.
3- Department of Sports Sciences, Faculty of Literature and Humanities, Kerman Branch, Islamic Azad University, Kerman, Iran.
Full-Text [PDF 5330 kb]
(224 Downloads)
| Abstract (HTML) (431 Views)
Full-Text: (280 Views)
Introduction
Muscle atrophy can be related to some physical injuries. Decreased muscle function is one of the most important factors related to decreased mobility which affects the quality of life (QoL). Although the mechanisms involved in muscle atrophy are not fully understood, it is believed that disruption of intracellular calcium homeostasis can play a role in the development of muscle function [1]. At the beginning of muscle contraction, calcium is released from the sarcoplasmic reticulum into the cytosol through ryanodine and dihydropyridine receptors, and at the end of the contraction, calcium is returned to the sarcoplasmic reticulum. During each phase of contraction, a fraction of calcium is released through the membrane calcium pump. Accordingly, maintaining the functional levels of calcium inside the sarcoplasmic reticulum and the influx of calcium-regulated by the cytosol across the plasma membrane is important for the function of muscle fibers in different stages of growth and response to stressful situations. Store-operated calcium entry (SOCE) is a mechanism for the entry of extracellular calcium into the cell in response to calcium released from intracellular stores [2] and plays an important role in controlling calcium homeostasis [3]. Many studies have related the role of SOCE to growth, proliferation, and apoptotic processes in many cells. In situations where the need for calcium increases, SOCE acts as a passageway for calcium entry to adapt to the increased need for calcium-dependent processes in muscle fibers [1]. Research evidence shows that calcium signaling pathways in skeletal muscles are dependent on incoming calcium and SOCE is important to maintain calcium to prevent muscle weakness and provide calcium needed to modulate the expression of muscle-specific genes [4].
Several proteins are involved in the coordination of SOCE. Stromal interaction molecule 1 (STIM1) is a calcium sensor located in the sarcoplasmic reticulum. It has been shown that mice lacking STIM1 have weak muscles and both tetanic and stimulated forces are reduced in the state of fatigue [4]. STIM1 protein is involved in controlling the entry of calcium ions into cells when calcium levels are low, especially through calcium release-activated calcium channel protein 1 (CRAC). The flow of calcium ions through CRAC causes intracellular signals that play an important role in many cellular functions, such as controlling gene activity, cell growth, and division, as well as immune function [5]. When calcium levels in the endoplasmic reticulum are low, changes occur in the cell that enables STIM1 to bind to the protein ORAI1 (calcium conductor in the T muscle system) in the cell membrane. ORAI1 protein, which is part of CRAC, creates a hole in the cell membrane through which calcium ions can flow [6]. Also, the mitsugumin 29 (MG29) protein, which is a structural protein, seems to be expressed exclusively in skeletal muscle (in the transverse tubes and terminal cisterna in the sarcoplasmic reticulum) and is involved in calcium influx [7]. MG29 protein is a muscle-specific member of the synaptophysin family, which is involved in controlling the maturation and development of transverse tubule structure and maintaining intracellular calcium signals in skeletal muscle. Genetic destruction of MG29 leads to incomplete formation of transverse tube network in skeletal muscles [8]. In addition, mice lacking the MG29 gene show abnormal performance in skeletal muscles, such as low contractile force and impaired SOCE. In chronic muscle-wasting diseases or disorders, such as atrophy and sarcopenia, mice lacking the MG29 gene are more prone to fatigue [9].
Sports activity can improve sports and muscle performance by creating different physiological adaptations [10]. It is also presented as an effective intervention in reducing the loss of muscle mass and function [2]. The ability of muscle fibers to respond to external stimuli is considered muscle variability. Various intracellular changes, such as changes in metabolites, hypoxia, mechanical stress, and free intracellular calcium are involved in these changes. It has been shown that calcium cell cycle changes with muscle activity are the most abundant and potential secondary messengers in skeletal muscles [11]. Intracellular calcium plays an important role in muscle fibers to produce force, maintaining the cell’s energy supply, regulating the expression of muscle-specific genes, and the process of apoptosis [12]. It has been shown that aerobic exercises improve the movement of calcium in skeletal muscles and are associated with the improvement of sports activity and skeletal muscle performance. In this regard, it has been stated that sports activity increases the levels of proteins involved in the release and uptake of calcium in the sarcoplasmic reticulum [13]. In addition, an increase in dihydropyridine gene and protein expression has been observed in response to muscle activity [14]. Based on this, understanding the mechanisms involved in muscle atrophy and the role of genes involved in calcium flow is important for the development of new treatment methods to deal with conditions and diseases, such as muscular dystrophy and atrophy caused by inactivity. Accordingly, the present study aims to investigate the effect of reduced physical activity after a combined training period on the expression of STIM1, ORAI1, and MG29 genes in the plantaris muscle of male Wistar rats.
Materials and Methods
In this experimental study, a total of 16 adult male Wistar rats in the weight range of 250±20 g were selected. To get familiar with the environment of the animal house, the rats were kept in the animal laboratory at a temperature of 22±4°C under a 12/12 h dark-light cycle and were fed with special food and water. Throughout the research, the mice were moved and manipulated by two individuals, and all the processes of the present research were carried out following all the ethical principles of working with animals.
Research groups
Animal samples were randomly divided into two groups. The groups were divided as follows: 1) Control group-spinal nerve ligation (SNL) (8 rats), 2) Combined training group–SNL (8 rats). At the beginning of the research, the animals of the combined exercise-SNL group participated in the exercise program (combined resistance and endurance exercise) for 6 weeks. The animals of the control-combined-SNL group did not perform any sports activities during this period. Subsequently, the SNL protocol was implemented for 4 weeks in both research groups. In the end, the mice were dissected and tissue samples were taken to perform cellular and molecular tests.
Combined exercise program (endurance-resistance)
The combined training program in this research was performed as follows: The animals of the training group performed a combination of endurance and resistance training. At the beginning and before the start of the endurance training program, the animals walked for 5 days a week for 10 to 15 min at a speed of 10 m/min on the treadmill for rodents. For endurance training in the present study, moderate training intensity (60% to 70% of maximum oxygen consumption) was used. Thus, the animals of the training group were subjected to endurance training on the treadmill for 6 weeks and 3 sessions every week. The speed and duration of training increased gradually (Table 1).

To achieve the obtained adaptations to a uniform state, all training variables were kept constant in the final week (sixth week) [15].
Resistance training was in the form of climbing a ladder for rodents with weights. In the familiarization phase with resistance training, the mice were introduced to how to climb the ladder for 10 to 15 min daily for 3 days. The main exercise was performed with target weights connected to the mouse’s tail through the cylinder and the mouse climbed the ladder in this state. This exercise was performed for six weeks by climbing a 1-m ladder with weights at an 85-degree incline. Training sessions were held twice in the morning (at 9:00), in the evening (at 14:00), and once every 3 days. The rats performed 3 sets of 5 repetitions in each session. The rest interval between sets was 2 min while it was 1 min between repetitions. When necessary, electric shock (0.2-0.3 mA) was used to stimulate the rats to climb the ladder. To increase the load, in the first week, 50% of the body weight of the mice was used and the load was gradually increased during the training program.
Physical activity reduction protocol using spinal nerve ligation model
The SNL model is a method that is widely used to study the mechanisms of neuropathic pain and the effect of drugs and behaviors related to pain. To create an SNL model, the rats were first anesthetized with sodium pentobarbital (60 mg per kg of body weight; intraperitoneally), and then their fifth lumbar spinal nerve was ligated and tightly tied according to the method of Kim and Chung (1992) [17]. The duration of the spinal cord ligation protocol was 4 weeks. In this method, after ensuring that the animal is anesthetized, the intervertebral muscles at the level of the fourth lumbar and second sacral vertebrae were separated and the transverse appendage of the sixth lumbar vertebra was removed. Then, the fifth lumbar nerve on the left side of the spinal cord was separated from the adjacent nerves with special delicacy and firmly tied using special thread silk (made in Japan), exactly at the distal end to ensure disruption of all fibers. This method was performed with great precision to prevent damage to the fourth lumbar nerve.
Tissue extraction method
After 4 weeks of ligation, the rats were anesthetized and immediately weighed. Then, under completely sterile conditions using a surgical blade, the plantaris muscle was extracted by cutting the proximal and distal tendons and weighed with a laboratory scale (accuracy 0.0001; AND GR model made in Japan), immediately frozen in liquid nitrogen, and kept in the freezer until the cell-molecular tests.
RNA extraction and cDNA synthesis
About 50 mg of plantaris muscle tissue was homogenized in QIAzol lysis reagent at a ratio of 1: 10 to extract total RNA. To remove the protein components, the product was centrifuged at 12000 g for 10 min at 4°C and then mixed with chloroform at a ratio of 0.5: 1 and shaken vigorously for 15 s. The product was centrifuged at 4°C, 15 min, and 12000 g, and the mineral and aqueous fractions were separated. Then, the part containing RNA was removed and mixed with isopropanol at a ratio of 0.5: 1 and left for 10 min at room temperature and then centrifuged at 12000 g for 10 min at 4°C. Next, the pellet containing RNA was washed in ethanol and dissolved in 20 μL of RNAS-free water. Also, the RNA concentration was measured (using the Eppendorf device, Germany) and the ratio of 260 to 280 between 1.8 and 2 was defined as the optimal purity. cDNA synthesis was done using 1 µg of RNA and using the cDNA synthesis kit to make fermentate and Mulv reverse transcriptase enzyme.
Real-time polymerase chain reaction
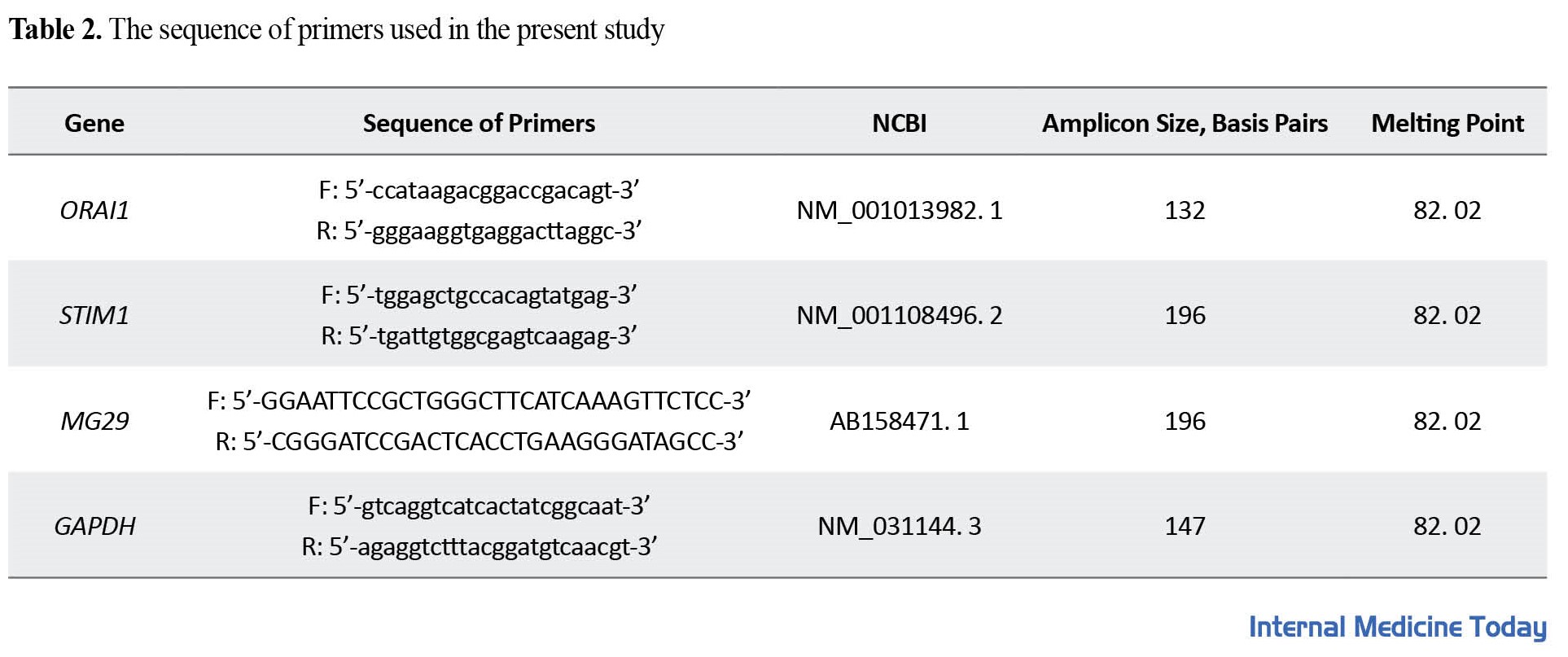
The measurement of the expression levels of the desired genes in the present study was done by quantitative real-time-PCR method using the Primix cyber green II (USA Applied Biosystems). The reaction mixture was in a final volume of 20 µL and each reaction was performed in duplicate. Primers were designed based on the information of genes in the NCBI gene bank and by Macrogen Inc. Seoul, Korea. The sequence of primers used in the research is reported in Table 2.
In addition, GAPDH was used as a control gene. The temperature program used in the real time-PCR included 95°C for 10 min, 95°C for 15 s, and 60°C for 1 min (repetition of 40 cycles). The expression level of the desired genes was also measured by the CT∆∆-2 method.
Data analysis
Descriptive statistics were used to describe the data and inferential statistics were used to test the research hypotheses. In the inferential statistics section, the Kolmogorov-Smirnov and Levene tests were used to check the normality of the data along with the homogeneity of the variances. After verifying these assumptions, to determine the significance of the difference in gene expression, an independent t-test was used at a significance level of 0. 05 using the SPSS software, version 20.
Results
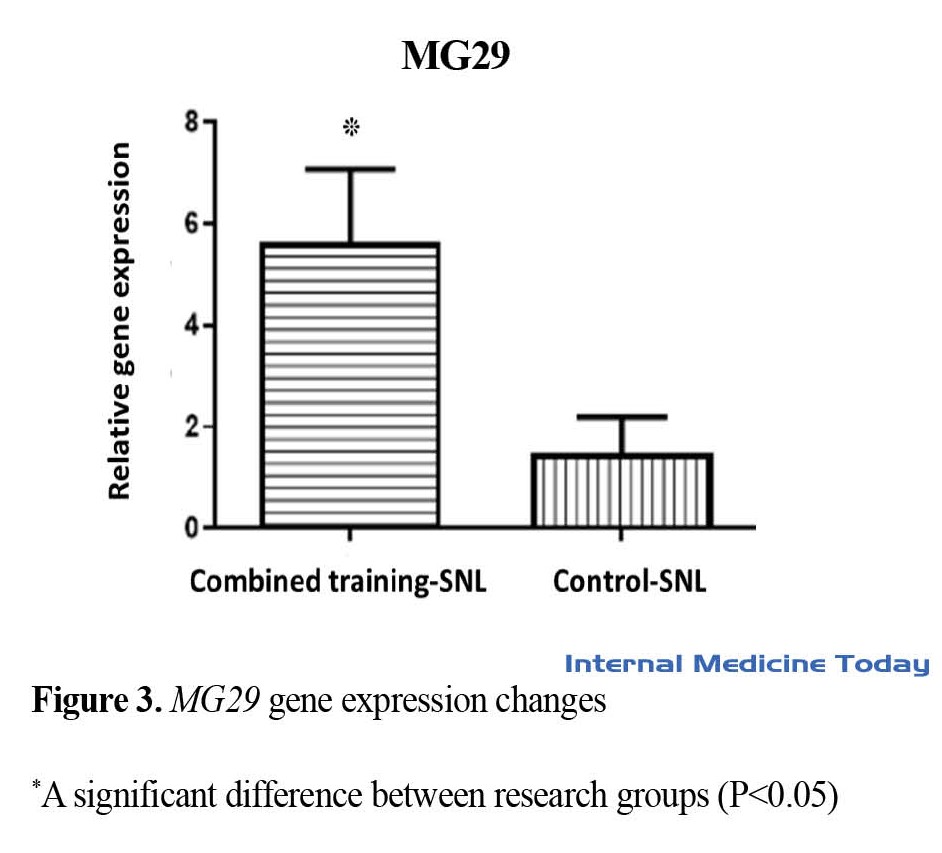
The results of the independent t test showed a significant effect of the exercise factor on the STIM1 gene expression levels in the plantaris muscle in the combined exercise-SNL group compared to the control group-SNL (P=0.01). Accordingly, performing a combined training period before the SNL leads to an increase in STIM1 gene expression in the plantaris muscle (Figure 1). Also, a significant effect of the training factor on ORAI1 gene expression levels in the plantaris muscle was observed in the combined exercise-SNL group compared to the control group-SNL (P=0.002). Therefore, performing a combined training session before SNL leads to an increase in ORAI1 gene expression in the plantaris muscle (Figure 2). In addition, a significant effect of the exercise factor on MG29 gene expression levels in the plantaris muscle was observed in the combined exercise-SNL group compared to the control group-SNL (P=0.02). Therefore, performing a combined training period before SNL leads to an increase in MG29 gene expression in the plantaris muscle (Figure 3).
Discussion
Atrophy of muscle mass occurs in conditions of long-term non-use of organs. Some physiological (without mechanical loading of organs) and pathological conditions (disturbance in blood supply) lead to atrophy and loss of muscle mass [18, 19]. In this situation, the decrease in power production and atrophy can affect the QoL of people [20]. Therefore, it is important to discover the mechanisms related to muscle atrophy to prevent and treat this phenomenon. In the current study, the effect of reduced physical activity in the form of SNL after a period of combined training on the expression of genes involved in calcium flow was evaluated. The results of the research showed that an increase in the expression of STIM1, ORAI1, and MG29 genes was observed in the combined exercise-SNL group compared to the control group-SNL. These results show that SNL leads to a decrease in the expression of these genes in the plantaris muscle, and performing a combined training session before SNL has a compensatory effect in this regard.
Intracellular calcium ions represent a signaling mediator that plays an important role in regulating various cellular processes in muscles. Maintaining calcium homeostasis is essential for maintaining the structure and function of skeletal muscles. SOCE, which is activated by the depletion of intracellular calcium stores (helps regulate various functions in many cell types) is critical for ensuring proper calcium homeostasis in muscle cells and is coordinated by STIM1 and ORAI1. The influx of calcium through SOCE plays an important role in the short-term and long-term functions of muscles, regulation, and adaptation of many cellular processes, including muscle contraction, postnatal growth, phenotype, and plasticity of myofibers. The loss or mutation of STIM1/ORAI1 genes and changes in SOCE are associated with serious consequences for muscle function. Also, evidence shows that changes in SOCE can cause changes in intracellular calcium signals in skeletal muscle, which are involved in the pathogenesis of various progressive muscle diseases, such as tubular myopathy, muscular dystrophy, cachexia, and sarcopenia [21]. It has been reported that new junctions between the sarcoplasmic reticulum and transverse tubules formed during exercise, which contain STIM1 and ORAI1, act as calcium entry units. These junctions provide a path for the rapid recovery of calcium ions from the extracellular space during repetitive muscle activity [22]. The MG29 protein is involved in controlling the maturation and expansion of the structure of the transverse tubes and maintaining intracellular calcium signals in skeletal muscle. Genetic destruction of MG29 leads to incomplete formation of transverse tube network in skeletal muscles. The amount of MG29 protein decreases significantly in animal models with muscular dystrophy, and the reduced expression of MG29 and the disturbed structure of the transverse tubules have been seen in human samples suffering from muscular dystrophy [8].
As a result of reducing physical activity (without muscle loading), the gene and protein expression of TRPC1 and TRPC3 channels decreases in the skeletal muscles of rats, and with reloading, their expression returns to the initial state. Considering the role of these channels in muscle growth, the observed changes in TRPC1 and TRPC3 may be closely related to the processes of muscle atrophy and regeneration [23]. Also, in the investigation of the functional role and effect of painful nerve damage on calcium influx stored in sensory nerve cells, axon damage due to SNL increases SOCE and CRAC. However, when calcium stores were depleted, SOCE was similar in injured and control neurons, and STIM1 and ORAI1 levels were not altered by SNL. These results show that the reset of SOCE after nerve ligation is done by the depletion of calcium stores. Also, suppression of SOCE increases the excitability of neurons in control conditions as well as damaged neurons. This is while damaged nerve cells show special dependence on SOCE to maintain cytoplasmic levels and store calcium. This dependence shows the compensatory role of SOCE after nerve damage [24].
Some researchers have investigated the effect of exercise on homeostasis and calcium influx. For example, Izadi et al. (2017) showed that intense intermittent exercise in diabetic rats improves the inappropriate regulation of RyR2 using a mechanism that targets the intensity and duration of exercise, and the type of exercise normalizes or reduces the effect of the reduction of RyR2 gene expression and its inappropriate function caused by diabetic cardiomyopathy [25]. In addition, it has been shown that the expression of cellular calcium regulatory genes, such as STIM1 and ORAI1, is significantly reduced by moderate-intensity endurance exercise and improves intracellular calcium signals in liver lymphocytes [26]. These results are consistent with the present study. Also, in line with the findings of the present study, Sazvar et al. (2016), investigating the effect of intense intermittent exercise on RyR2 calcium channels and calcium pump in ischemia rats showed that the expression of SERCA2a gene increased in both exercise and exercise-ischemia groups. It was found that this increase was significantly higher in the exercise-ischemia group, and intense intermittent exercise increased RyR2 gene expression in both exercise-ischemia and trained groups which can eliminate abnormal contractions associated with cardiomyopathy caused by ischemia of the heart muscle and regulate the flow of calcium in the heart muscle [27]. In addition, Pecorai et al. (2018) in their study, titled “the effect of exercise training in the form of repetitive electrical stimulation in old mice with condensed transverse tubular myopathy disease,” which leads to the accumulation of STIM1 and ORAI1 in an ineffective form showed that exercise limits the formation of this disease and improves muscle function [28]. In examining the effect of endurance training on myogenic responses and ion flow caused by muscle atrophy, the possible increase in hemodynamic stimuli plays an important role in maintaining the myogenic response and calcium ion flow from TRPC channels. This explains the positive effect of endurance training in patients with limb paralysis because endurance training reduces myogenic response and calcium ion currents caused by atrophy [29]. Edwards et al. (2010) in the study of the effect of aerobic training on calcium influx associated with metabolic syndrome and coronary atherosclerosis showed that this training method has a protective effect on calcium influx by reducing the expression of TRPC1 and STIM1 genes and leads to decreased calcium influx associated with metabolic syndrome and coronary atherosclerosis [30].
Conclusion
According to the results of the present research, it was found that SNL leads to a decrease in the expression of STIM1, ORAI1, and MG29 genes in the plantaris muscle, and performing a combined training session before SNL has a compensatory effect, which is probably one of the mechanisms related to the beneficial effects of exercise training on calcium homeostasis. However, more research is needed to clarify the main mechanisms in this regard. Performing a combination exercise was one of the strengths of the present study because this type of exercise can bring different answers and adaptations compared to other exercise programs. One of the limitations of the current research was the lack of evaluation of genes related to muscle atrophy.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the ethics committee of Kerman University of Medical Sciences (Code: IR.KMU.REC.1399.190).
Funding
This article was extracted from the master’s thesis of Sobhan Naghizadeh, registered by the Department of Sports Sciences, Islamic Azad University, Kerman Branch. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors have contributed equally to the preparation of this article.
Conflicts of interest
The authors declared no conflict of interest.
References
Muscle atrophy can be related to some physical injuries. Decreased muscle function is one of the most important factors related to decreased mobility which affects the quality of life (QoL). Although the mechanisms involved in muscle atrophy are not fully understood, it is believed that disruption of intracellular calcium homeostasis can play a role in the development of muscle function [1]. At the beginning of muscle contraction, calcium is released from the sarcoplasmic reticulum into the cytosol through ryanodine and dihydropyridine receptors, and at the end of the contraction, calcium is returned to the sarcoplasmic reticulum. During each phase of contraction, a fraction of calcium is released through the membrane calcium pump. Accordingly, maintaining the functional levels of calcium inside the sarcoplasmic reticulum and the influx of calcium-regulated by the cytosol across the plasma membrane is important for the function of muscle fibers in different stages of growth and response to stressful situations. Store-operated calcium entry (SOCE) is a mechanism for the entry of extracellular calcium into the cell in response to calcium released from intracellular stores [2] and plays an important role in controlling calcium homeostasis [3]. Many studies have related the role of SOCE to growth, proliferation, and apoptotic processes in many cells. In situations where the need for calcium increases, SOCE acts as a passageway for calcium entry to adapt to the increased need for calcium-dependent processes in muscle fibers [1]. Research evidence shows that calcium signaling pathways in skeletal muscles are dependent on incoming calcium and SOCE is important to maintain calcium to prevent muscle weakness and provide calcium needed to modulate the expression of muscle-specific genes [4].
Several proteins are involved in the coordination of SOCE. Stromal interaction molecule 1 (STIM1) is a calcium sensor located in the sarcoplasmic reticulum. It has been shown that mice lacking STIM1 have weak muscles and both tetanic and stimulated forces are reduced in the state of fatigue [4]. STIM1 protein is involved in controlling the entry of calcium ions into cells when calcium levels are low, especially through calcium release-activated calcium channel protein 1 (CRAC). The flow of calcium ions through CRAC causes intracellular signals that play an important role in many cellular functions, such as controlling gene activity, cell growth, and division, as well as immune function [5]. When calcium levels in the endoplasmic reticulum are low, changes occur in the cell that enables STIM1 to bind to the protein ORAI1 (calcium conductor in the T muscle system) in the cell membrane. ORAI1 protein, which is part of CRAC, creates a hole in the cell membrane through which calcium ions can flow [6]. Also, the mitsugumin 29 (MG29) protein, which is a structural protein, seems to be expressed exclusively in skeletal muscle (in the transverse tubes and terminal cisterna in the sarcoplasmic reticulum) and is involved in calcium influx [7]. MG29 protein is a muscle-specific member of the synaptophysin family, which is involved in controlling the maturation and development of transverse tubule structure and maintaining intracellular calcium signals in skeletal muscle. Genetic destruction of MG29 leads to incomplete formation of transverse tube network in skeletal muscles [8]. In addition, mice lacking the MG29 gene show abnormal performance in skeletal muscles, such as low contractile force and impaired SOCE. In chronic muscle-wasting diseases or disorders, such as atrophy and sarcopenia, mice lacking the MG29 gene are more prone to fatigue [9].
Sports activity can improve sports and muscle performance by creating different physiological adaptations [10]. It is also presented as an effective intervention in reducing the loss of muscle mass and function [2]. The ability of muscle fibers to respond to external stimuli is considered muscle variability. Various intracellular changes, such as changes in metabolites, hypoxia, mechanical stress, and free intracellular calcium are involved in these changes. It has been shown that calcium cell cycle changes with muscle activity are the most abundant and potential secondary messengers in skeletal muscles [11]. Intracellular calcium plays an important role in muscle fibers to produce force, maintaining the cell’s energy supply, regulating the expression of muscle-specific genes, and the process of apoptosis [12]. It has been shown that aerobic exercises improve the movement of calcium in skeletal muscles and are associated with the improvement of sports activity and skeletal muscle performance. In this regard, it has been stated that sports activity increases the levels of proteins involved in the release and uptake of calcium in the sarcoplasmic reticulum [13]. In addition, an increase in dihydropyridine gene and protein expression has been observed in response to muscle activity [14]. Based on this, understanding the mechanisms involved in muscle atrophy and the role of genes involved in calcium flow is important for the development of new treatment methods to deal with conditions and diseases, such as muscular dystrophy and atrophy caused by inactivity. Accordingly, the present study aims to investigate the effect of reduced physical activity after a combined training period on the expression of STIM1, ORAI1, and MG29 genes in the plantaris muscle of male Wistar rats.
Materials and Methods
In this experimental study, a total of 16 adult male Wistar rats in the weight range of 250±20 g were selected. To get familiar with the environment of the animal house, the rats were kept in the animal laboratory at a temperature of 22±4°C under a 12/12 h dark-light cycle and were fed with special food and water. Throughout the research, the mice were moved and manipulated by two individuals, and all the processes of the present research were carried out following all the ethical principles of working with animals.
Research groups
Animal samples were randomly divided into two groups. The groups were divided as follows: 1) Control group-spinal nerve ligation (SNL) (8 rats), 2) Combined training group–SNL (8 rats). At the beginning of the research, the animals of the combined exercise-SNL group participated in the exercise program (combined resistance and endurance exercise) for 6 weeks. The animals of the control-combined-SNL group did not perform any sports activities during this period. Subsequently, the SNL protocol was implemented for 4 weeks in both research groups. In the end, the mice were dissected and tissue samples were taken to perform cellular and molecular tests.
Combined exercise program (endurance-resistance)
The combined training program in this research was performed as follows: The animals of the training group performed a combination of endurance and resistance training. At the beginning and before the start of the endurance training program, the animals walked for 5 days a week for 10 to 15 min at a speed of 10 m/min on the treadmill for rodents. For endurance training in the present study, moderate training intensity (60% to 70% of maximum oxygen consumption) was used. Thus, the animals of the training group were subjected to endurance training on the treadmill for 6 weeks and 3 sessions every week. The speed and duration of training increased gradually (Table 1).

To achieve the obtained adaptations to a uniform state, all training variables were kept constant in the final week (sixth week) [15].
Resistance training was in the form of climbing a ladder for rodents with weights. In the familiarization phase with resistance training, the mice were introduced to how to climb the ladder for 10 to 15 min daily for 3 days. The main exercise was performed with target weights connected to the mouse’s tail through the cylinder and the mouse climbed the ladder in this state. This exercise was performed for six weeks by climbing a 1-m ladder with weights at an 85-degree incline. Training sessions were held twice in the morning (at 9:00), in the evening (at 14:00), and once every 3 days. The rats performed 3 sets of 5 repetitions in each session. The rest interval between sets was 2 min while it was 1 min between repetitions. When necessary, electric shock (0.2-0.3 mA) was used to stimulate the rats to climb the ladder. To increase the load, in the first week, 50% of the body weight of the mice was used and the load was gradually increased during the training program.
Physical activity reduction protocol using spinal nerve ligation model
The SNL model is a method that is widely used to study the mechanisms of neuropathic pain and the effect of drugs and behaviors related to pain. To create an SNL model, the rats were first anesthetized with sodium pentobarbital (60 mg per kg of body weight; intraperitoneally), and then their fifth lumbar spinal nerve was ligated and tightly tied according to the method of Kim and Chung (1992) [17]. The duration of the spinal cord ligation protocol was 4 weeks. In this method, after ensuring that the animal is anesthetized, the intervertebral muscles at the level of the fourth lumbar and second sacral vertebrae were separated and the transverse appendage of the sixth lumbar vertebra was removed. Then, the fifth lumbar nerve on the left side of the spinal cord was separated from the adjacent nerves with special delicacy and firmly tied using special thread silk (made in Japan), exactly at the distal end to ensure disruption of all fibers. This method was performed with great precision to prevent damage to the fourth lumbar nerve.
Tissue extraction method
After 4 weeks of ligation, the rats were anesthetized and immediately weighed. Then, under completely sterile conditions using a surgical blade, the plantaris muscle was extracted by cutting the proximal and distal tendons and weighed with a laboratory scale (accuracy 0.0001; AND GR model made in Japan), immediately frozen in liquid nitrogen, and kept in the freezer until the cell-molecular tests.
RNA extraction and cDNA synthesis
About 50 mg of plantaris muscle tissue was homogenized in QIAzol lysis reagent at a ratio of 1: 10 to extract total RNA. To remove the protein components, the product was centrifuged at 12000 g for 10 min at 4°C and then mixed with chloroform at a ratio of 0.5: 1 and shaken vigorously for 15 s. The product was centrifuged at 4°C, 15 min, and 12000 g, and the mineral and aqueous fractions were separated. Then, the part containing RNA was removed and mixed with isopropanol at a ratio of 0.5: 1 and left for 10 min at room temperature and then centrifuged at 12000 g for 10 min at 4°C. Next, the pellet containing RNA was washed in ethanol and dissolved in 20 μL of RNAS-free water. Also, the RNA concentration was measured (using the Eppendorf device, Germany) and the ratio of 260 to 280 between 1.8 and 2 was defined as the optimal purity. cDNA synthesis was done using 1 µg of RNA and using the cDNA synthesis kit to make fermentate and Mulv reverse transcriptase enzyme.
Real-time polymerase chain reaction
The measurement of the expression levels of the desired genes in the present study was done by quantitative real-time-PCR method using the Primix cyber green II (USA Applied Biosystems). The reaction mixture was in a final volume of 20 µL and each reaction was performed in duplicate. Primers were designed based on the information of genes in the NCBI gene bank and by Macrogen Inc. Seoul, Korea. The sequence of primers used in the research is reported in Table 2.

In addition, GAPDH was used as a control gene. The temperature program used in the real time-PCR included 95°C for 10 min, 95°C for 15 s, and 60°C for 1 min (repetition of 40 cycles). The expression level of the desired genes was also measured by the CT∆∆-2 method.
Data analysis
Descriptive statistics were used to describe the data and inferential statistics were used to test the research hypotheses. In the inferential statistics section, the Kolmogorov-Smirnov and Levene tests were used to check the normality of the data along with the homogeneity of the variances. After verifying these assumptions, to determine the significance of the difference in gene expression, an independent t-test was used at a significance level of 0. 05 using the SPSS software, version 20.
Results
The results of the independent t test showed a significant effect of the exercise factor on the STIM1 gene expression levels in the plantaris muscle in the combined exercise-SNL group compared to the control group-SNL (P=0.01). Accordingly, performing a combined training period before the SNL leads to an increase in STIM1 gene expression in the plantaris muscle (Figure 1). Also, a significant effect of the training factor on ORAI1 gene expression levels in the plantaris muscle was observed in the combined exercise-SNL group compared to the control group-SNL (P=0.002). Therefore, performing a combined training session before SNL leads to an increase in ORAI1 gene expression in the plantaris muscle (Figure 2). In addition, a significant effect of the exercise factor on MG29 gene expression levels in the plantaris muscle was observed in the combined exercise-SNL group compared to the control group-SNL (P=0.02). Therefore, performing a combined training period before SNL leads to an increase in MG29 gene expression in the plantaris muscle (Figure 3).
Discussion
Atrophy of muscle mass occurs in conditions of long-term non-use of organs. Some physiological (without mechanical loading of organs) and pathological conditions (disturbance in blood supply) lead to atrophy and loss of muscle mass [18, 19]. In this situation, the decrease in power production and atrophy can affect the QoL of people [20]. Therefore, it is important to discover the mechanisms related to muscle atrophy to prevent and treat this phenomenon. In the current study, the effect of reduced physical activity in the form of SNL after a period of combined training on the expression of genes involved in calcium flow was evaluated. The results of the research showed that an increase in the expression of STIM1, ORAI1, and MG29 genes was observed in the combined exercise-SNL group compared to the control group-SNL. These results show that SNL leads to a decrease in the expression of these genes in the plantaris muscle, and performing a combined training session before SNL has a compensatory effect in this regard.
Intracellular calcium ions represent a signaling mediator that plays an important role in regulating various cellular processes in muscles. Maintaining calcium homeostasis is essential for maintaining the structure and function of skeletal muscles. SOCE, which is activated by the depletion of intracellular calcium stores (helps regulate various functions in many cell types) is critical for ensuring proper calcium homeostasis in muscle cells and is coordinated by STIM1 and ORAI1. The influx of calcium through SOCE plays an important role in the short-term and long-term functions of muscles, regulation, and adaptation of many cellular processes, including muscle contraction, postnatal growth, phenotype, and plasticity of myofibers. The loss or mutation of STIM1/ORAI1 genes and changes in SOCE are associated with serious consequences for muscle function. Also, evidence shows that changes in SOCE can cause changes in intracellular calcium signals in skeletal muscle, which are involved in the pathogenesis of various progressive muscle diseases, such as tubular myopathy, muscular dystrophy, cachexia, and sarcopenia [21]. It has been reported that new junctions between the sarcoplasmic reticulum and transverse tubules formed during exercise, which contain STIM1 and ORAI1, act as calcium entry units. These junctions provide a path for the rapid recovery of calcium ions from the extracellular space during repetitive muscle activity [22]. The MG29 protein is involved in controlling the maturation and expansion of the structure of the transverse tubes and maintaining intracellular calcium signals in skeletal muscle. Genetic destruction of MG29 leads to incomplete formation of transverse tube network in skeletal muscles. The amount of MG29 protein decreases significantly in animal models with muscular dystrophy, and the reduced expression of MG29 and the disturbed structure of the transverse tubules have been seen in human samples suffering from muscular dystrophy [8].
As a result of reducing physical activity (without muscle loading), the gene and protein expression of TRPC1 and TRPC3 channels decreases in the skeletal muscles of rats, and with reloading, their expression returns to the initial state. Considering the role of these channels in muscle growth, the observed changes in TRPC1 and TRPC3 may be closely related to the processes of muscle atrophy and regeneration [23]. Also, in the investigation of the functional role and effect of painful nerve damage on calcium influx stored in sensory nerve cells, axon damage due to SNL increases SOCE and CRAC. However, when calcium stores were depleted, SOCE was similar in injured and control neurons, and STIM1 and ORAI1 levels were not altered by SNL. These results show that the reset of SOCE after nerve ligation is done by the depletion of calcium stores. Also, suppression of SOCE increases the excitability of neurons in control conditions as well as damaged neurons. This is while damaged nerve cells show special dependence on SOCE to maintain cytoplasmic levels and store calcium. This dependence shows the compensatory role of SOCE after nerve damage [24].
Some researchers have investigated the effect of exercise on homeostasis and calcium influx. For example, Izadi et al. (2017) showed that intense intermittent exercise in diabetic rats improves the inappropriate regulation of RyR2 using a mechanism that targets the intensity and duration of exercise, and the type of exercise normalizes or reduces the effect of the reduction of RyR2 gene expression and its inappropriate function caused by diabetic cardiomyopathy [25]. In addition, it has been shown that the expression of cellular calcium regulatory genes, such as STIM1 and ORAI1, is significantly reduced by moderate-intensity endurance exercise and improves intracellular calcium signals in liver lymphocytes [26]. These results are consistent with the present study. Also, in line with the findings of the present study, Sazvar et al. (2016), investigating the effect of intense intermittent exercise on RyR2 calcium channels and calcium pump in ischemia rats showed that the expression of SERCA2a gene increased in both exercise and exercise-ischemia groups. It was found that this increase was significantly higher in the exercise-ischemia group, and intense intermittent exercise increased RyR2 gene expression in both exercise-ischemia and trained groups which can eliminate abnormal contractions associated with cardiomyopathy caused by ischemia of the heart muscle and regulate the flow of calcium in the heart muscle [27]. In addition, Pecorai et al. (2018) in their study, titled “the effect of exercise training in the form of repetitive electrical stimulation in old mice with condensed transverse tubular myopathy disease,” which leads to the accumulation of STIM1 and ORAI1 in an ineffective form showed that exercise limits the formation of this disease and improves muscle function [28]. In examining the effect of endurance training on myogenic responses and ion flow caused by muscle atrophy, the possible increase in hemodynamic stimuli plays an important role in maintaining the myogenic response and calcium ion flow from TRPC channels. This explains the positive effect of endurance training in patients with limb paralysis because endurance training reduces myogenic response and calcium ion currents caused by atrophy [29]. Edwards et al. (2010) in the study of the effect of aerobic training on calcium influx associated with metabolic syndrome and coronary atherosclerosis showed that this training method has a protective effect on calcium influx by reducing the expression of TRPC1 and STIM1 genes and leads to decreased calcium influx associated with metabolic syndrome and coronary atherosclerosis [30].
Conclusion
According to the results of the present research, it was found that SNL leads to a decrease in the expression of STIM1, ORAI1, and MG29 genes in the plantaris muscle, and performing a combined training session before SNL has a compensatory effect, which is probably one of the mechanisms related to the beneficial effects of exercise training on calcium homeostasis. However, more research is needed to clarify the main mechanisms in this regard. Performing a combination exercise was one of the strengths of the present study because this type of exercise can bring different answers and adaptations compared to other exercise programs. One of the limitations of the current research was the lack of evaluation of genes related to muscle atrophy.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the ethics committee of Kerman University of Medical Sciences (Code: IR.KMU.REC.1399.190).
Funding
This article was extracted from the master’s thesis of Sobhan Naghizadeh, registered by the Department of Sports Sciences, Islamic Azad University, Kerman Branch. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors have contributed equally to the preparation of this article.
Conflicts of interest
The authors declared no conflict of interest.
References
- Zhao X, Weisleder N, Thornton A, Oppong Y, Campbell R, Ma J, et al. Compromised store-operated Ca2+ entry in aged skeletal muscle. Aging Cell. 2008; 7(4): 561-8. [DOI: 10. 1111/j. 1474-9726. 2008. 00408. x] [PMID] [PMCID]
- Edwards JN, Blackmore DG, Gilbert DF, Murphy RM, Launikonis BS. Store-operated calcium entry remains fully functional in aged mouse skeletal muscle despite a decline in STIM1 protein expression. Aging Cell. 2011; 10(4): 675-85. [DOI: 10. 1111/j. 1474-9726. 2011. 00706. x] [PMID]
- Zhao X, Min CK, Ko JK, Parness J, Kim DH, Weisleder N, et al. Increased store-operated Ca2+ entry in skeletal muscle with reduced calsequestrin-1 expression. Biophysical Journal. 2010; 99(5): 1556-64. [DOI: 10. 1016/j. bpj. 2010. 06. 050] [PMID] [PMCID]
- Rosenberg PB. Calcium entry in skeletal muscle. The Journal of Physiology. 2009; 587(Pt 13): 3149-51. [DOI: 10. 1113/jphysiol. 2009. 172585] [PMID] [PMCID]
- Lee KJ, Hyun C, Woo JS, Park CS, Kim DH, Lee EH. Stromal interaction molecule 1 (STIM1) regulates sarcoplasmic/endoplasmic reticulum Ca 2+-ATPase 1a (SERCA1a) in skeletal muscle. Pflügers Archiv: European Journal of Physiology. 2014; 466(5): 987-1001. [DOI: 10. 1007/s00424-013-1361-6] [PMID]
- Nesin V, Wiley G, Kousi M, Ong EC, Lehmann T, Nicholl DJ, et al. Activating mutations in STIM1 and ORAI1 cause overlapping syndromes of tubular myopathy and congenital miosis. Proceedings of the National Academy of Sciences. 2014; 111(11): 4197-202. [DOI: 10. 1073/pnas. 1312520111] [PMID] [PMCID]
- Shimuta M, Komazaki S, Nishi M, Iino M, Nakagawara K, Takeshima H. Structure and expression of mitsugumin29 gene. FEBS Letters. 1998; 431(2): 263-7. [DOI: 10. 1016/S0014-5793(98)00770-4] [PMID]
- Jin F, Choi K-H, Ko J-K, Park K-H, Cheng C, Yao X, et al. Mir181A Targets the 3’UTR of MG29, a Muscle-Specific Synaptophysin Family Gene, for Down-Regulation of MG29 Expression in Dystrophic Skeletal Muscle. Biophysical Journal. 2015; 108(2): 590a. [DOI: 10. 1016/j. bpj. 2014. 11. 3216]
- Woo JS, Hwang JH, Huang M, Ahn MK, Cho CH, Ma J, et al. Interaction between mitsugumin 29 and TRPC3 participates in regulating Ca2+ transients in skeletal muscle. Biochemical and Biophysical Research Communications. 2015; 464(1): 133-9. [DOI: 10. 1016/j. bbrc. 2015. 06. 096] [PMID] [PMCID]
- Gibala MJ, Little JP, van Essen M, Wilkin GP, Burgomaster KA, Safdar A, et al. Short-term sprint interval versus traditional endurance training: Similar initial adaptations in human skeletal muscle and exercise performance. The Journal of Physiology. 2006; 575(3): 901-11. [DOI: 10. 1113/jphysiol. 2006. 112094] [PMID] [PMCID]
- Gundersen K. Excitation-transcription coupling in skeletal muscle: The molecular pathways of exercise. Biological Reviews of The Cambridge Philosophical Society. 2011; 86(3): 564-600. [DOI: 10. 1111/j. 1469-185X. 2010. 00161. x] [PMID] [PMCID]
- Chin ER. Intracellular Ca2+ signaling in skeletal muscle: decoding a complex message. Exercise and Sport Sciences Reviews. 2010; 38(2): 76-85. [DOI: 10. 1097/JES. 0b013e3181d495d2] [PMID]
- Bueno Jr CR, Ferreira JCB, Pereira MG, Bacurau AV, Brum PC. Aerobic exercise training improves skeletal muscle function and Ca2+ handling-related protein expression in sympathetic hyperactivity-induced heart failure. Journal of Applied Physiology. 2010; 109(3): 702-9. [DOI: 10. 1152/japplphysiol. 00281. 2010] [PMID]
- Song W, Kwak HB, Lawler JM. Exercise training attenuates age-induced changes in apoptotic signaling in rat skeletal muscle. Antioxidants & Redox Signaling. 2006; 8(3-4): 517-28. [DOI: 10. 1089/ars. 2006. 8. 517] [PMID]
- Chae CH, Kim HT. Forced, moderate-intensity treadmill exercise suppresses apoptosis by increasing the level of NGF and stimulating phosphatidylinositol 3-kinase signaling in the hippocampus of induced aging rats. Neurochemistry International. 2009; 55(4): 208-13. [DOI: 10. 1016/j. neuint. 2009. 02. 024] [PMID]
- Lee S, Farrar RP. Resistance training induces muscle-specific changes in muscle mass and function in rat. Journal of Exercise Physiology online. 2003; 6(2). [Link]
- Ho Kim S, Mo Chung J. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992; 50(3): 355-63. [DOI: 10. 1016/0304-3959(92)90041-9] [PMID]
- Hodges P, Holm AK, Hansson T, Holm S. Rapid atrophy of the lumbar multifidus follows experimental disc or nerve root injury. Spine. 2006; 31(25): 2926-33. [DOI: 10. 1097/01. brs. 0000248453. 51165. 0b] [PMID]
- Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: A brief review. Neurosurgical Focus. 2004; 16(5): E1. [DOI: 10. 3171/foc. 2004. 16. 5. 2] [PMID]
- Fanzani A, Conraads VM, Penna F, Martinet W. Molecular and cellular mechanisms of skeletal muscle atrophy: An update. Journal of Cachexia, Sarcopenia and Muscle. 2012; 3(3): 163-79. [DOI: 10. 1007/s13539-012-0074-6] [PMID] [PMCID]
- Conte E, Imbrici P, Mantuano P, Coppola MA, Camerino GM, De Luca A, et al. Alteration of STIM1/Orai1-Mediated SOCE in Skeletal Muscle: Impact in genetic muscle diseases and beyond. Cells. 2021; 10(10): 2722. [DOI: 10. 3390/cells10102722] [PMID] [PMCID]
- Boncompagni S, Michelucci A, Pietrangelo L, Dirksen RT, Protasi F. Exercise-dependent formation of new junctions that promote STIM1-Orai1 assembly in skeletal muscle. Scientific Reports. 2017; 7(1): 14286. [DOI: 10. 1038/s41598-017-14134-0] [PMID] [PMCID]
- Zhang BT, Yeung SS, Cheung KK, Chai ZY, Yeung EW. Adaptive responses of TRPC1 and TRPC3 during skeletal muscle atrophy and regrowth. Muscle & Nerve. 2014; 49(5): 691-9. [DOI: 10. 1002/mus. 23952] [PMID]
- Gemes G, Bangaru MLY, Wu HE, Tang Q, Weihrauch D, Koopmeiners AS, et al. Store-operated Ca2+ entry in sensory neurons: Functional role and the effect of painful nerve injury. Journal of Neuroscience. 2011; 31(10): 3536-49. [DOI: 10. 1523/JNEUROSCI. 5053-10. 2011] [PMID] [PMCID]
- Izadi MR, Gaeini AA, Ravasi AA, Delfan M. [Effect of 4 weeks high intensity interval training on gene expression of Ryanodine receptor calcium channels (RyR2), SERCA2a and Phospholamban in diabetic rat’s heart (Persian)]. Journal of Sport Biosciences. 2018; 10(1): 1-12. [Link]
- Liu R, Fan W, Krüger K, Xiao Y, Pilat C, Seimetz M, et al. Exercise affects T-Cell function by modifying intracellular calcium homeostasis. Medicine and Science in Sports and Exercise. 2017; 49(1): 29-39. [DOI: 10. 1249/MSS. 0000000000001080] [PMID]
- Sazvar A, Mehrialvar Y, Ghardashi AA, Nazari MH. [The effect of eight weeks of interval training on gene expression of ryanodine receptors’calcium channels and calcium pump in ischemic rats (Persian)]. J Sabzevar Univ Med Sci. 2018; 25(2): 89-98. [Link]
- Pecorai C, Michelucci A, Pietrangelo L, Protasi F, Boncompagni S. Exercise prevents formation of tubular aggregates in ageing skeletal muscle fibers of wild-type mice. Biophysical Journal. 2018; 114(3): 470A. [DOI: 10. 1016/j. bpj. 2017. 11. 2588]
- Yin MZ, Kim HJ, Suh EY, Zhang YH, Yoo HY, Kim SJ. Endurance exercise training restores atrophy-induced decreases of myogenic response and ionic currents in rat skeletal muscle artery. Journal of Applied Physiology. 2019; 126(6): 1713-24. [DOI: 10. 1152/japplphysiol. 00962. 2018] [PMID]
- Edwards JM, Neeb ZP, Alloosh MA, Long X, Bratz IN, Peller CR, et al. Exercise training decreases store-operated Ca2+ entry associated with metabolic syndrome and coronary atherosclerosis. Cardiovascular Research. 2010; 85(3): 631-40. [DOI: 10. 1093/cvr/cvp308] [PMID] [PMCID]
Type of Study: Original |
Subject:
Physiology
Received: 2022/05/13 | Accepted: 2022/09/23 | Published: 2022/09/23
Received: 2022/05/13 | Accepted: 2022/09/23 | Published: 2022/09/23
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |